Using 2D nanomaterials to create electric vehicle fuel

MXenes are 2D nanomaterials just a few atoms thick. First discovered in 2011, MXenes have a wide range of potential uses, from batteries to sensors to biomedical applications. Babak Anasori, Reilly Rising Star Professor of Materials and Mechanical Engineering, has been designing novel MXenes in his labs, both in Indianapolis and West Lafayette.
“Many scientists, including myself and my group, have been looking for the best MXene to use in the hydrogen evolution reaction (HER) process,” stated Anasori.
His team recently discovered a new MXene using the element tungsten, which is synthesized much differently than typical ones and can be used as an electrocatalyst to speed up the HER process. This process is what generates hydrogen fuel, which can then be used in fuel cells for electric vehicles, potentially solving commonly faced issues like range and charging time.
Their research has been published in Nature Synthesis.
The tungsten MXene was chosen because it was predicted to be the most efficient catalyst in the HER process, offering high reactivity and minimum potential required to perform the action of splitting water into hydrogen and oxygen. Tungsten is also readily abundant in the earth’s crust.
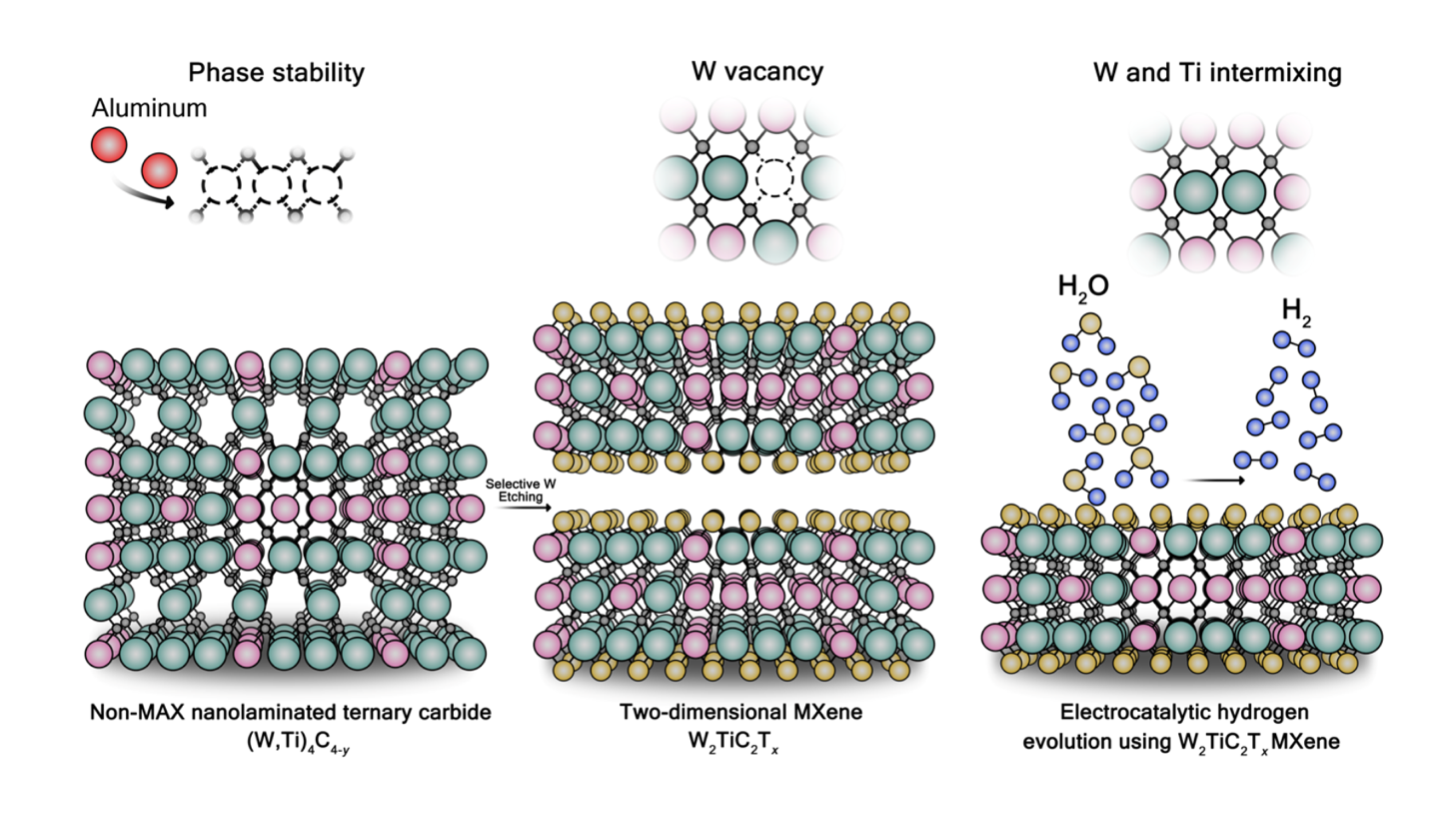
The process of designing a typical MXene involves mixing a transition metal, aluminum, and carbon together to form a MXene precursor, known as the MAX phase. Then the atomic layers of aluminum are selectively removed through a process called etching which utilizes acids like hydrofluoric acid.
This time, Anasori and team created a different precursor by mixing tungsten, titanium, carbon, and aluminum. By adding the excess amount of aluminum, they were able to control the arrangement of tungsten and titanium atoms so that when they etched the sample, they could pull out certain atomic layers of tungsten.
“We use hydrofluoric acid to selectively remove the tungsten layers,” explained Anupma Thakur, postdoctoral research associate in Anasori’s lab. “This etching process happens over 4 days at 55 degrees Celsius and leaves us with a multi-layered structure, which we then delaminate to synthesize single-to-few layered 2D MXene sheets.”
Moving forward, Babak and team hope to find more use cases for this tungsten MXene, a new member of a growing 2D MXene family. They also want to experiment with other metals to see if they can design more MXenes with other potential applications.
“There are many different directions we want to take, there’s no end to our curiosity,” said Anasori.
This research has been conducted with support from the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under Award DE-SC0017717 and the National Science Foundation (NSF) under the DMREF award 10002504.
Source: Babak Anasori, banasori@purdue.edu
Writer: Julia Davis, juliadavis@purdue.edu
Synthesis of a 2D tungsten MXene for electrocatalysis
Anupma Thakur, Wyatt J. Highland, Brian C. Wyatt, Jiayi Xu, Nithin Chandran B.S., Bowen Zhang, Zachary D. Hood, Shiba P. Adhikari, Emad Oveisi, Barbara Pacakova, Fernando Vega, Jeffrey Simon, Colton Fruhling, Benjamin Reigle, Mohammad Asadi, Pawe P. Michaowski, Vladimir M. Shalaev, Alexandra Boltasseva, Thomas E. Beechem, Cong Liu, Babak Anasori
https://www.nature.com/articles/s44160-025-00773-z
ABSTRACT: Two-dimensional transition metal carbides, nitrides, and carbonitrides, known as MXenes, are of interest as electrocatalysts. Tungsten- (W) based MXenes are predicted to have low overpotentials in the hydrogen evolution reaction (HER), however, their synthesis has proven difficult due to the calculated instability of its hypothetical MAX precursors. In this study, we present a theory-guided synthesis of a W-based MXene, W2TiC2Tx, derived from a non-MAX nanolaminated ternary carbide (W,Ti)4C4-y precursor by the selective etching of one of the covalently bonded tungsten layers. Our results indicate the importance of W and Ti ordering, the presence of vacancy defects in the metal layers, and lack of oxygen impurities in the carbon layers for the successful selective etching of the precursor. We confirm the atomistic out-of-plane ordering of W and Ti using computational and experimental characterizations. The W-rich basal plane endows W2TiC2TxMXene with high electrocatalytic HER performance (overpotential ~144 mV at 10 mA/cm2). This study reports a W-based MXene synthesized from a covalently bonded non-MAX precursor, adding to the synthetic strategies for 2D materials.
