Safety at the core: probing the reliability of solid-state batteries

Lithium-ion batteries have three key components: an anode, a cathode, and an electrolyte in the middle, which is typically a flammable liquid or gel. If the battery overheats, the electrolyte can pose a fire hazard. The Internet is full of dramatic images showing cars, laptops, or cell phones in flames due to an overheated battery.
Solid-state batteries replace the liquid electrolyte with a solid-state electrolyte, which is not flammable. In theory, this would make the battery much safer, and simultaneously provide greater energy density due to the utilization of a lithium metal anode. But this is only part of the story.
“Solid-state batteries hold tremendous promise for advancing electromobility; but at the same time, a wide range of scientific and engineering challenges need to be addressed. Among these, safety is an important consideration,” said Bairav S. Vishnugopi, a Ph.D. student in Mechanical Engineering studying under Prof. Partha Mukherjee.
It’s not just the electrolyte that is changed in a solid-state battery. The anode, which is typically graphite in a lithium-ion battery, is replaced with lithium metal. “The physical contact between the lithium anode and the solid electrolyte can lead to chemical decomposition of the solid electrolyte, forming a resistive layer at the interface,” said Vishnugopi. “We call these interphases. Their chemical composition and evolution play a decisive role on how much heat is generated in the battery.”
Mukherjee’s team – including Bairav S. Vishnugopi, Md Toukir Hasan and Hanwei Zhou – performed a detailed study of the thermal stability and interphase-mediated interactions in solid-state batteries, and laid out an extensive safety landscape for future research in solid-state batteries. Their research has been published in ACS Energy Letters, and featured as an Energy Spotlight (Editor’s Choice) article.
The team evaluated the thermal response of three different sulfide-based solid electrolytes: Li3PS4 (LPS), Li6PS5Cl (LPSCl), and Li10SnP2S12 (LSPS). Using accelerating rate calorimetry experiments, they were able to examine the thermal stability of the interface between the lithium anode and the solid electrolyte. Compared to LPS and LPSCl, a drastically different thermal runaway behavior was observed in LSPS owing to its dynamically growing interphase. Complementary to these calorimetry experiments, X-ray photoelectron spectroscopy (located at Birck Nanotechnology Center) demonstrated the unique link between the chemical composition of the interphase and the thermal signature.
“The thermal runaway observed in case of the LSPS electrolyte is dictated by the evolving nature of its interphase. Intriguingly, after electrochemical cycling, the LSPS/lithium interface exhibits a massive thermal spike near the melting point of lithium,” said Vishnugopi.
The paper presents another important insight. Mukherjee’s team developed a thermo-kinetic model to understand the effects of the anode-electrolyte’s thermal response in a full cell configuration. Their model revealed that the heat released at the anode interface can induce oxygen release at the cathode, which in turn could cause more interphase reactions at the anode, spiraling into a thermal runaway event. “This ‘electrode crosstalk’ is a crucial safety consideration for solid-state batteries, especially due to the propensity for oxygen release at the cathode that can lead to a severe exothermic interaction with the lithium anode,” said Prof. Partha Mukherjee.
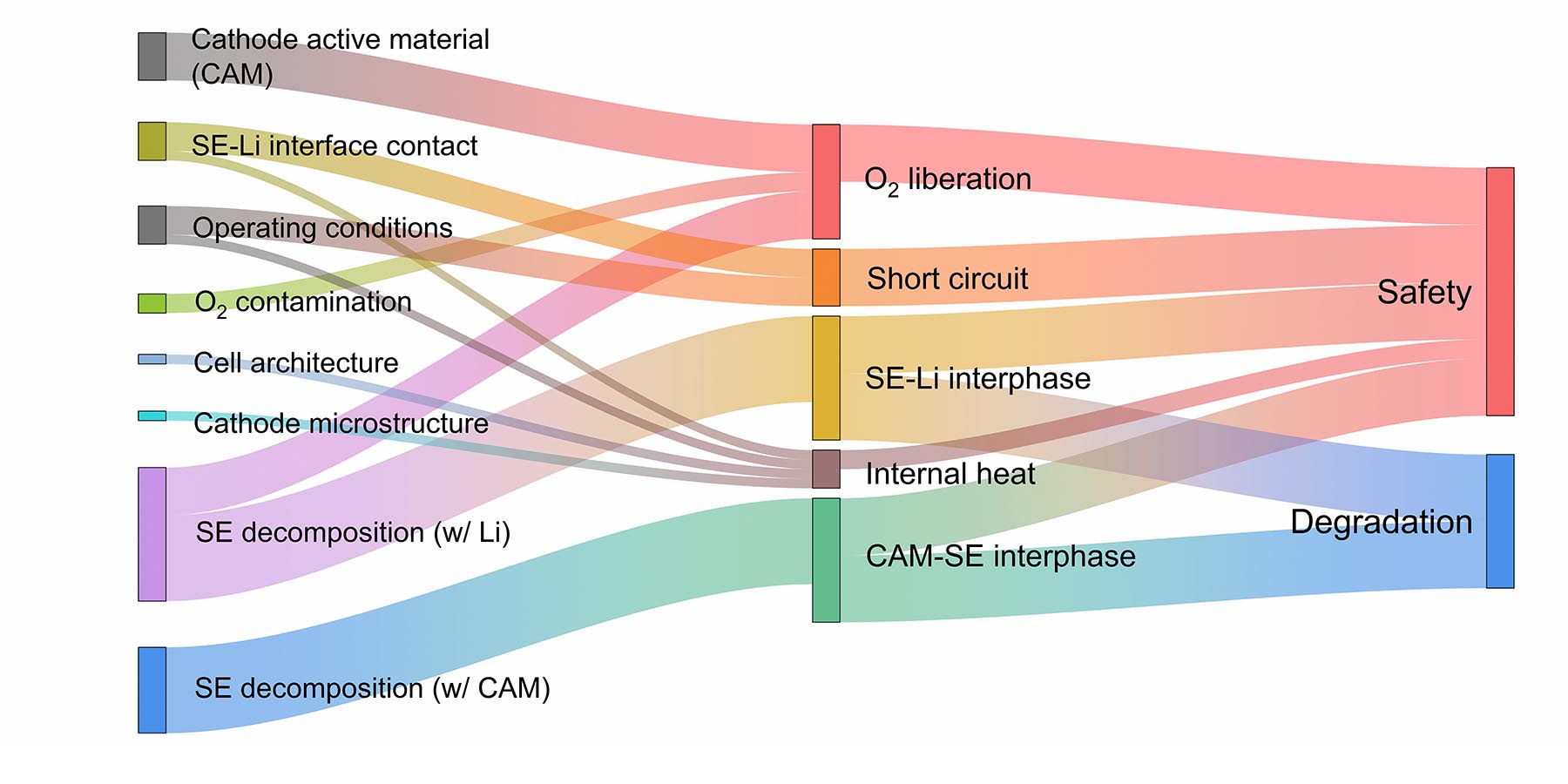
A final goal of the paper is to lay out the thermal stability landscape for solid-state batteries: what mechanistic interactions occur at solid-solid interfaces and how they are connected to resulting safety and degradation behavior. Beyond the canonical identification of solid-state battery safety in terms of nonflammability, Mukherjee’s team aims to build a comprehensive correlation between the electro-chemo-mechanics of solid-solid interfaces and their thermal stability, which can pave the way for a scale-bridging safety analysis in future.
“As solid-state batteries are in relatively early stages of their development, it would be judicious to synergistically analyze their safety attributes while we progress toward achieving the performance metrics required for various applications,” said Prof. Mukherjee.
This research is supported in part by the Office of Naval Research (N00014-22-1-2516).
Writer: Jared Pike, jaredpike@purdue.edu, 765-496-0374
Source: Partha Mukherjee, pmukherjee@purdue.edu, 765-494-2219
Interphases and Electrode Crosstalk Dictate the Thermal Stability of Solid-State Batteries
Bairav S. Vishnugopi, Md Toukir Hasan, Hanwei Zhou, and Partha P. Mukherjee
https://doi.org/10.1021/acsenergylett.2c02443
ABSTRACT: Solid-state batteries, because of their high energy density, are promising candidates for long-range electric vehicles and electric aviation. While the enhanced safety potential of solid-state batteries has been typically ascribed to the nonflammability of solid electrolytes, an extensive interrogation of their thermal stability is still required. In this work, we reveal how the thermal stability in sulfide-based solid-state batteries is critically dependent on the interphase interactions at the solid electrolyte/Li interface, thereby illustrating the drastically different thermal signature of Li10SnP2S12 when compared with Li3PS4 and Li6PS5Cl. Our study shows that thermal runaway occurs even for a pristine Li10SnP2S12/Li interface and is severely exacerbated with cycling, which exhibits a massive thermal spike at the melting point of Li; this shift in thermal response uniquely correlates to the Li10SnP2S12 interphase evolution. On the basis of these distinct thermal signatures, cell-level mechanistic safety maps cognizant of the Li/interphase interaction, cathode/Li crosstalk, and specific energy are delineated.
