Inkjet-printed tumors: custom cancer drug testbeds in less than a day
“Drug discovery is an important aspect of cancer research,” said Bumsoo Han, professor of mechanical engineering. “But to develop a new cancer drug, they typically have to test about 10,000 different chemicals. Every test needs some sort of live tumor model in order to fully test its effectiveness. These tumors take time to create and validate.”
Han specializes in mimicking these tumor environments with microfluidic devices — in one case, watching pancreatic cancer disappear as if in a time machine. But when it comes to creating actual 3D tumoroids (tumors custom-created in a lab, rather than naturally in the body), there aren’t a lot of options.
“There have been advances in tissue engineering, trying to 3D print these tumoroids,” Han said. “But it’s very challenging to achieve the cell density you need to model real human tumors. The resolution of 3D printing is not great, and it also takes a very long time — sometimes weeks for just one tumoroid to mature. We wanted to find a way to accelerate the process.”
They decided to experiment with inkjet printing, rather than conventional 3D printing.
“Normal inkjet printing on paper is far more precise than most people realize,” said George Chiu, professor of mechanical engineering. “Even the cheapest inkjet printers can spray precise individual dots of just 20 microns in diameter, using one-trillionth of a liter of ink. That speed and precision is impossible for even the best 3D printer.”
So how do they convert 2D inkjet-printed biomaterial into a 3D tumoroid? In this case, they let the cells themselves do all the work.

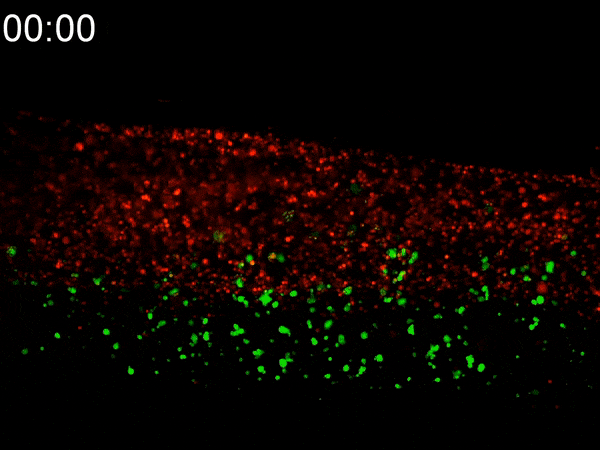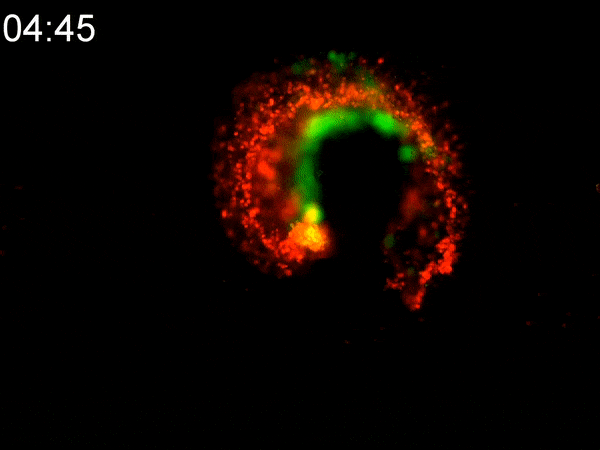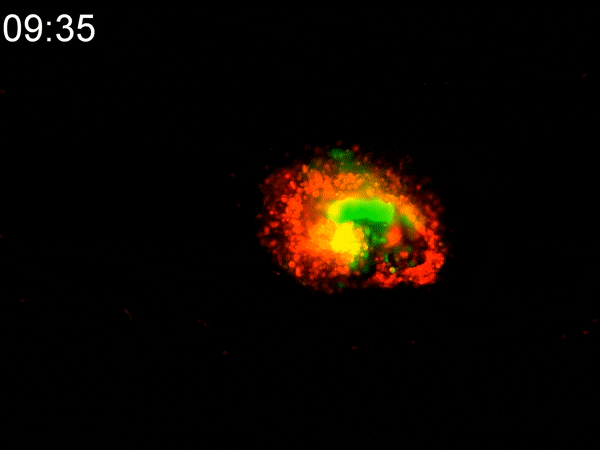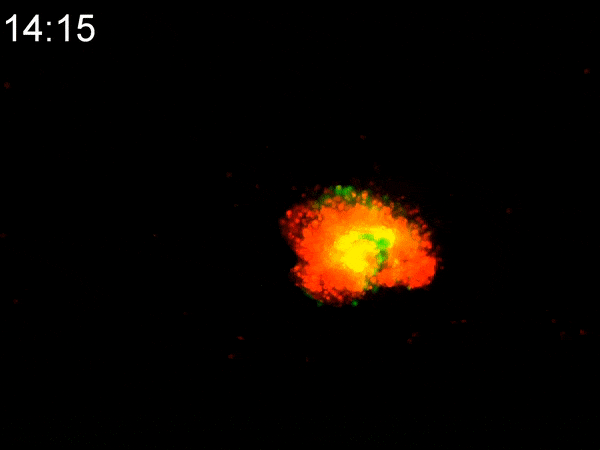
Using a custom-built inkjet printer, researchers print two different types of cells simultaneously, each with different geometric and mechanical properties. By printing them in a specific pattern, the cells’ different contractile forces cause the 2D shape to fold in on itself, creating a dense 3D tumoroid in just a matter of hours. While existing bioprinting techniques require days or weeks to properly culture a 3D-printed tumoroid, this flat inkjet-printed tumoroid will morph into a finished 3D shape in less than a day.
Their research has been published in Materials Today Advances.
Key to this research is incorporating not just the cancer cells, but also the surrounding tissue that connects them (called the stroma). “There are many biochemical and biomechanical interactions between the cancer cells and the stroma,” Han said. “The stromal component is critically important to create properly engineered tumor models.”
To experiment with these two types of cells, Cih Cheng, the paper’s lead author (who earned her Ph.D. in 2022), helped to develop a custom inkjet-printing platform with two print heads. One contained a hydrogel with cancerous cells; the other with cancer-associated fibroblasts (CAF), acting as the stromal tissue. She printed the two different cells in a specific pattern, spread over an area several millimeters square. Within hours, the 2D shape had begun to curl up. In less than a day, they had a 3D tumoroid about one-half millimeter in diameter that had the same cell density and characteristics of tumors found in vivo.
To explain how this happens, Chiu uses an analogy from thermostats: “A thermostat functions with something called a bimetallic strip, where each side is a different metal,” he said. “As the temperature changes, one metal expands and one contracts, causing the strip to bend in one direction to complete the circuit. That’s exactly what’s happening here, except it’s two different kinds of cells with different material properties.”
They were shocked at how fast the morphogenesis (shape development) happened. “We expected that cell contraction might happen,” Han said. “But the speed was quite surprising to us. This is faster than any other manufacturing technique out there.
“The ability to custom print tumoroids in less than a day is a real game changer and will make drug discovery faster and more efficient.”
Other members of the research team included Chelsea Davis, assistant professor of materials engineering, and Bennett Elzey, research assistant professor of comparative pathobiology with the Purdue Institute for Cancer Research. This research is supported by grants from the National Cancer Institute and National Science Foundation.
The team have also secured a patent for its printing technology through the Purdue Innovates Office of Technology Commercialization. Industry partners interested in developing or commercializing the technology should contact Patrick Finnerty, pwfinnerty@prf.org, Senior Business Development and Licensing Manager – Life Sciences at OTC about 2020-HAN-68900.
The team is excited to continue investigating the possibilities of inkjet-printed morphogenesis, partnering with the Purdue Institute for Drug Discovery to immediately apply their creations into their drug discovery pipeline. “We are looking into new ink formulations, as well as new geometric patterns,” Chiu said. “If we can effectively utilize the cells’ own mechanical behavior, then we can use inkjet printing to quickly build all sorts of tissues. This is just the beginning.”
Writer: Jared Pike, jaredpike@purdue.edu, 765-496-0374
Sources: Bumsoo Han, bumsoo@purdue.edu
George Chiu, gchiu@purdue.edu
Inkjet-printed morphogenesis of tumor-stroma interface using bi-cellular bioinks of collagen-poly (N-isopropyl acrylamide-co-methyl methacrylate) mixture
Cih Cheng, Naomi Deneke, Hye-ran Moon, Sae Rome Choi, Natalia Ospina-Muñoz, Bennett D. Elzey, Chelsea S. Davis, George T.-C Chiu, Bumsoo Han
https://doi.org/10.1016/j.mtadv.2023.100408
ABSTRACT: Recent advances in biomaterials and 3D printing/culture methods enable various tissue-engineered tumor models. However, it is still challenging to achieve native tumor-like characteristics due to lower cell density than native tissues and prolonged culture duration for maturation. Here, we report a new method to create tumoroids with a mechanically active tumor-stroma interface at extremely high cell density. This method, named “inkjet-printed morphogenesis” (iPM) of the tumor-stroma interface, is based on a hypothesis that cellular contractile force can significantly remodel the cell-laden polymer matrix to form densely-packed tissue-like constructs. Thus, differential cell-derived compaction of tumor cells and cancer-associated fibroblasts (CAFs) can be used to build a mechanically active tumor-stroma interface. In this methods, two kinds of bioinks are prepared, in which tumor cells and CAFs are suspended respectively in the mixture of collagen and poly (N-isopropyl acrylamide-co-methyl methacrylate) solution. These two cellular inks are inkjet-printed in multi-line or multi-layer patterns. As a result of cell-derived compaction, the resulting structure forms tumoroids with mechanically active tumor-stroma interface at extremely high cell density. We further test our working hypothesis that the morphogenesis can be controlled by manipulating the force balance between cellular contractile force and matrix stiffness. Furthermore, this new concept of “morphogenetic printing” is demonstrated to create more complex structures beyond current 3D bioprinting techniques.
