LyoHUB2040 Technology Roadmap for Pharma/Biotech Manufacturing to be launched Oct. 4
On Oct. 4, Manufacturing Day, Purdue University plans to unveil a pivotal roadmap focusing on advancing pharmaceutical and biotechnology manufacturing. The roadmap will be launched as part of a groundbreaking event for The William D. and Sherry L. Young Institute for Advanced Manufacturing of Pharmaceuticals “racetrack” facility.
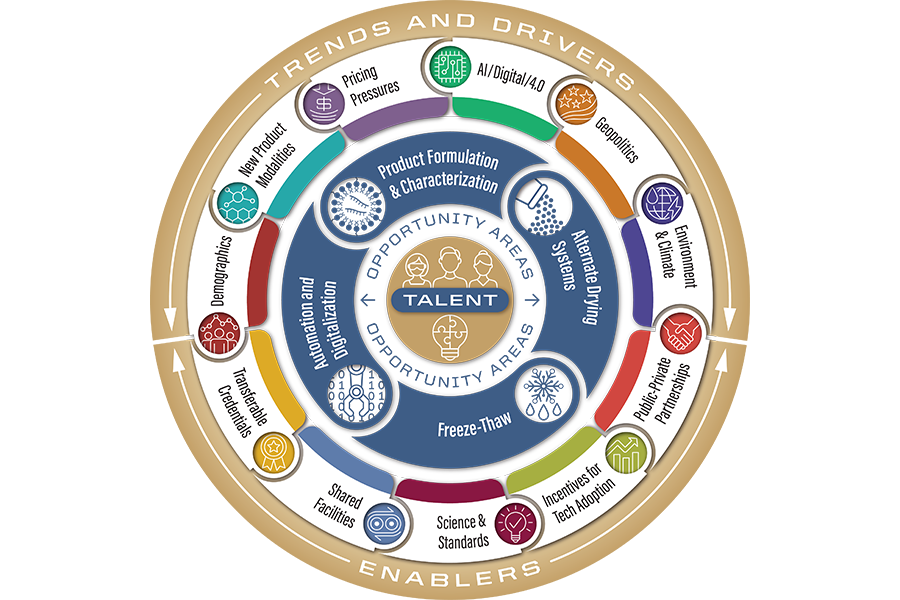
Over the past two years, the Advanced Lyophilization Technology Hub (LyoHUB), with support from a National Institute of Standards and Technology (NIST) Advanced Manufacturing Technology Roadmap (MFGTech) grant, has collaborated with more than 100 pharmaceutical and life sciences manufacturing experts to develop the Lyo2040: Lyophilization and Freeze-Thaw Technology Roadmap for Pharma/Biotech Manufacturing.
“Technology roadmaps are proven, strategic tools used to identify barriers and related development steps to achieve grand challenges,” according to NIST. “These (MFGTech) grants will enable essential first steps toward building the research infrastructure necessary to sustain a healthy, innovative advanced manufacturing sector — one that invents, demonstrates, prototypes and produces, here in the United States.”
The roadmap is designed to improve lyophilization, a process that preserves temperature-sensitive products such as vaccines and biologics. Lyophilization in its current state is a long and costly process.
“Our technology roadmap lays out ongoing research goals to improve process efficiency and cost-effectiveness and explore alternative drying techniques,” said Alina Alexeenko, LyoHUB director and professor of aeronautics and astronautics and chemical engineering. “This is especially vital as global conflicts and the recent coronavirus pandemic have increased demand for shelf-stable medicines and vaccines, driving home the need for strengthening domestic pharmaceutical manufacturing and the supply chain.”
The Lyo2040 roadmap builds on the first technology roadmap for lyophilization, the MFGTech-awarded Lyo2017 roadmap, to include novel freeze-drying, aseptic (contamination-free) drying, freeze-thaw technologies, new product treatments like cell and gene therapies, nucleotides (building blocks of DNA and RNA), vaccines and therapeutics, and education/workforce development. Advanced digital technologies will help achieve roadmap goals through automation and digitalization of the lyophilization process enabled by artificial intelligence, digital twins, big data, and data analytics.
“Technology roadmaps provide pathways to achieve real breakthroughs needed to innovate and manufacture new products, tools, and services,” said Lisa Jean Fronczek, Office of Advanced Manufacturing at NIST. “They are a great tool to bring academia and industry together to strengthen partnerships, advance U.S. competitiveness and develop future leadership for industries of tomorrow.”
Purdue’s role in Heartland Bioworks, one of 12 U.S. regional hubs for advancing biotech and national security, enhances its commitment to driving innovation and supporting biotech entrepreneurs. This involvement aligns with the One Health Innovation District’s focus on addressing health challenges across various domains, including animal, plant and human health through a partnership with Elanco Animal Health Inc. The Lyo2040: Lyophilization and Freeze-Thaw Technology Roadmap for Pharma/Biotech Manufacturing further integrates with these efforts by providing a strategic framework to advance pharmaceutical manufacturing, reinforcing Purdue’s leadership in biotech innovation and cutting-edge manufacturing technologies.
“The Lyo2040 Technology Roadmap represents a shared and aligned strategic vision for academia, technology developers and the pharmaceutical industry, to ensure development of innovative and impactful technologies to reduce cost, increase speed and maintain high quality science and pharmaceuticals,” said Nicholas Warne, Vice President of Pharmaceutical Research & Development at Pfizer.
The roadmap launch will take place at the groundbreaking of The William D. and Sherry L. Young Institute for Advanced Manufacturing of Pharmaceuticals “racetrack” facility at the Indiana Manufacturing Institute. LyoHUB will have a strong presence in this new research and training hub, which features a 10,000-square-foot manufacturing and training space, adding to Indiana’s growing pharmaceutical manufacturing footprint.
"This roadmap is critical to improving lyophilization technologies for shortening manufacturing times, enhancing quality, and bringing safer medication to patients more quickly and at a lower cost," said Eric Munson, scientific director of LyoHUB and Dane O. Kildsig Chair in Industrial and Physical Pharmacy Department Head.
