2023 Senior Design Projects
Madelyn Chadwick, Amanda Glime, Shaheer Faruqi, John Morris, and Shiv Shukla
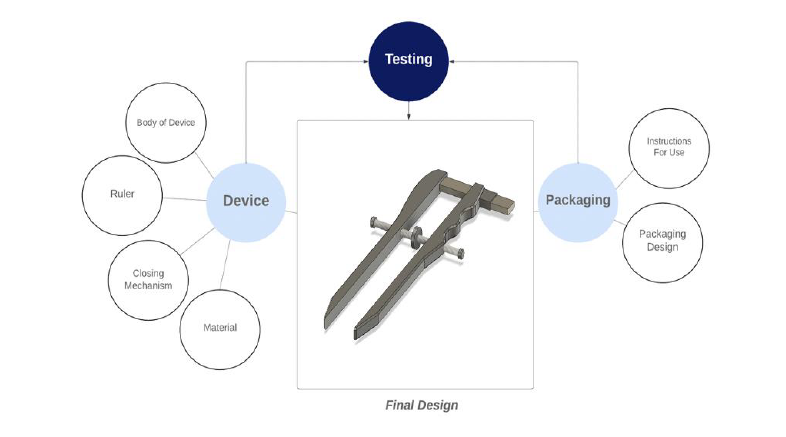
Peripheral nerve injuries occur at a frequency of 1 in every 1,000 people annually. Nerve conduits are becoming an increasingly popular treatment choice to bridge the gap between severed nerves as they create preferable microenvironment that enhances axonal regeneration after injury. Nerves are structures covered in layers of connective tissue and fat, with the diameter of each nerve varying from patient to patient. Accurate sizing of the nerve conduit to the nerve stump diameter is important for nerve recovery as a size mismatch can often lead to poor nerve regeneration, and decreased muscle reinnervation. Currently, a size mismatch between the nerve conduit and nerve conduit occurs in half of nerve injury repair procedures. This also leads to improper recovery which can cause pain and discomfort to the patients, consequently affecting their quality of life. Furthermore, there is an added cost of corrective surgeries which can be cost-prohibitive for the patients. To address these issues, we developed an intra-operative nerve-end diameter device to accurately measure the diameter of nerve ends during nerve injury repair. Our nerve measurement device draws inspiration from the operational mechanism of a caliper, incorporating straight arms that run parallel to each other. This innovative tool is designed for easy single-handed operation, featuring a thumb wheel that enables the surgeon to delicately narrow the device onto the nerve's ends. Once the desired measurement is achieved, a secure locking mechanism ensures stability and accuracy. A NIST-calibrated ruler is seamlessly integrated into the device, enhancing precision down to the nearest half millimeter. The straight arms within the device contribute significantly to its accuracy, ensuring that measurements at both ends are precisely identical. This unique design not only simplifies the measurement process but also provides unparalleled accuracy for nerve assessments, making it an indispensable tool in the medical field. When compared to traditional methods of measurement used to measure the diameter of nerve-ends, our device offers ergonomic usability, consistently provides accurate and precise measurements, and remains cost effective. Employing this device during peripheral nerve-injury repair procedure would allow surgeons to enhance the accuracy of nerve-end diameter measurements, promoting effective nerve regeneration and reducing the economic costs associated with corrective surgery.
Alexis Gies, Noah Grom, Grace Mathews, Madison Mullens, and Kaylee Czyzio
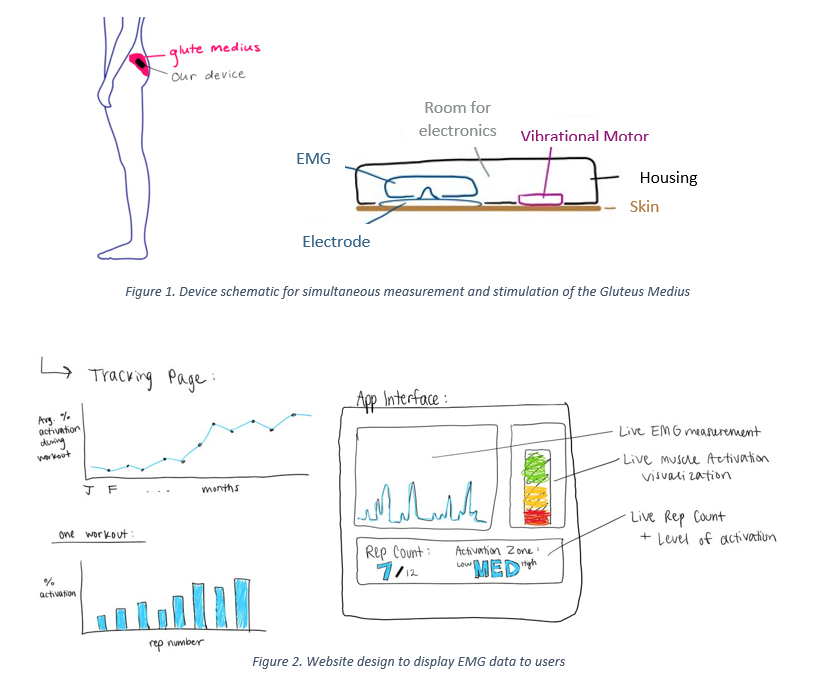
Patellofemoral pain syndrome (PFPS) is the leading cause of knee pain with incidence rates ranging between 9 – 15% amongst the general population. Weakness of the Gluteus Medius muscle causes excess knee valgus stress and lateral patellar displacement leading to the development of PFPS. The current standard of care for patients suffering from PFPS is physical therapy guided glute strengthening exercises. On average, patients meet with their physical therapist only one hour per week, and when patients perform the prescribed strengthening exercises at home, without the guidance of a PT, they are unable to properly activate the Gluteus Medius. Hence, there is a need for a cost-effective method (< $200) to address inadequate Gluteus Medius activation outside of PT-lead treatment in PFPS patients to increase long-term Gluteus Medius activation and decrease recurrence rates of PFPS. The proposed solution is a device comprised of a wireless EMG to monitor muscle activation and an ERM vibrational motor to provide a vibrational cue to the Gluteus Medius when muscle activation is below a specified threshold (Figure 1). The device is adhered, by electrode patches, to the skin over the Gluteus Medius muscle (Figure 1). A webpage displays the muscle activation data collected from the EMG to the user and PT (Figure 2). Current solutions in the treatment landscape for PFPS measure muscle activation or provide targeted muscle vibrational cues. However, no current device provides measurement and cues. Our solution is innovative because it provides stimulation of the muscle fibers based on simultaneous muscle activation measurements while also recording and displaying muscle activation data to the user. The solution increases the effectiveness of Gluteus Medius strengthening exercises, therefore shortening the overall duration of physical therapy for PFPS patients. Shorter duration of physical therapy contributes to decreased healthcare costs and increased quality of life for the patient.
Jillian Cawi, Elizabeth Grivetti, Maggie McGee, and Beth Ospalik

Automotive crashes are the leading cause of fetal mortality and maternal injury in the United States, accounting for over 50 percent of all trauma incurred during pregnancy and 83 percent of all fetal deaths. Pain and/or discomfort with the placement of the current universal three-point seat belt restraint across sensitive abdominal and breast tissue leads to incorrect usage in up to 87 percent of pregnant individuals. In the case of an accident, the improper placement of the seat belt restraint across the abdomen can lead to injuries such as placental abruption, uterine rupture, or laceration. With approximately 3.6 million pregnancies in the U.S. annually, an accessible and affordable solution to the disparity in accommodating pregnant anatomy must be addressed to ensure maternal and fetal safety in their everyday automotive travels. The V.I.P. Harness (Vehicle Injury Protection Harness) implements a lightweight, size-adjustable restraint system to accommodate variations in occupant body type and vehicular seat positions. This solution aims to provide an easy-to-use interchangeable system between vehicle seats while maintaining all federal regulations and safety standards. As no current solution landscape adequately meets both tissue sensitivity and adjustability needs, this device brings a new perspective and dedication to improving safety for typically underrepresented anatomy within the automotive industry.
Diya Sakhrani, Sathveka Sembian, Nikita Rao, Nick Buffo, and Maya Federle
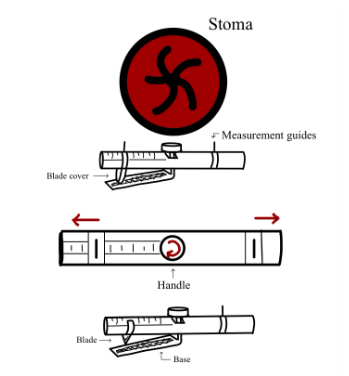
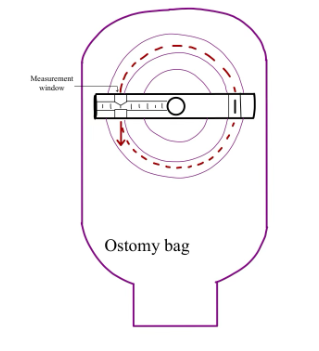
In the United States, over one million individuals currently live with a stoma due to the removal of diseased bowel. Up to 75% of these patients experience peristomal skin irritation due to leakage of stoma output onto the peristomal skin from ill-fitting ostomy bags. Currently, patients measure and cut their bags approximately every three days using a paper measuring guide and curved scissors. However, this multi-step system is time-consuming and does not provide precise sizing. Our proposed solution, Ostomeasure, would allow ostomy patients and medical providers to measure the stoma and cut an appropriately-sized hole in ostomy bags with one integrated device. The Ostomeasure presents a bilateral equal measurement system, a novel mechanism to measure the diameter of the stoma on a continuous measurement scale from 15 to 100 mm in diameter, thereby increasing accuracy of measurement. This design is innovative in that its one-sided guard allows the system to work in a one-piece and two-piece ostomy bag system. The device also measures the diameter of the stoma, but cuts using the radius. In addition, a measurement read-out will be provided so patients can visually identify quantitative changes in stoma size. This solution will increase cutting efficiency of bags, accessibility, and accuracy to mitigate stoma leakage and resulting skin irritation, thereby improving patient quality of life.
Charles King, Abhinav Krishnan, Shreya Krishnan, Richa Louis, and Andrew Wacker

Fractures of the radius and ulna are the most common fractures of the upper extremity, occurring in approximately 1 to 10 per 10,000 people per year. For most fractures, a forearm splint is used to immobilize the affected arm by preventing the pronation and supination of the forearm. Our project aims to innovate upon the current orthotic solutions for radial and ulnar fractures of the forearm to allow for a more comfortable, efficient, and optimized healing. Forearm splinting for radial and ulnar fractures in adults faces limitations such as insufficient personalization, excessive discomfort, constrained adaptability, intricate scanning processes, and elevated expenses. Thus, there is a need for a practical, prefabricated custom orthotic that helps establish proper healing habits for radial and ulnar fractures in young adults 16 to 40 years old. The solution must be as rigid as the current leading materials, able to be molded to custom orthotic levels, able to reshape after being molded to user, enhance user satisfaction, and the raw material should cost less than competing modern solutions. Our design is a flat 3D printed sheet of thermoplastic polyurethane that can be molded to custom specifications of a patient's arm. The custom orthotic would be molded by heating the sheet in 55 to 60 °C water and molding it to the patient’s forearm and hand. The orthotic will then cool and solidify creating a durable splint to protect and heal the forearm. Current solutions for the most efficient splints and other custom orthotics to treat radial and ulnar fractures in adults require the use of 3D scanners, require multiple visits to various medical professionals, and often result in less-than-optimal products due to the inability of the devices to easily adjust to the specific needs of the patient. We currently cater to patients in underserved communities, where medical resources are scarce and multiple hospital visits are not always feasible. By creating a custom orthotic, we can provide optimal care and facilitate efficient healing without increasing costs and hardship for the patient or medical provider.
Zein Chehab, Rama Coimbatore, Nikki Kulkarni, Radhika Kulkarni, and Tessa Wanthal
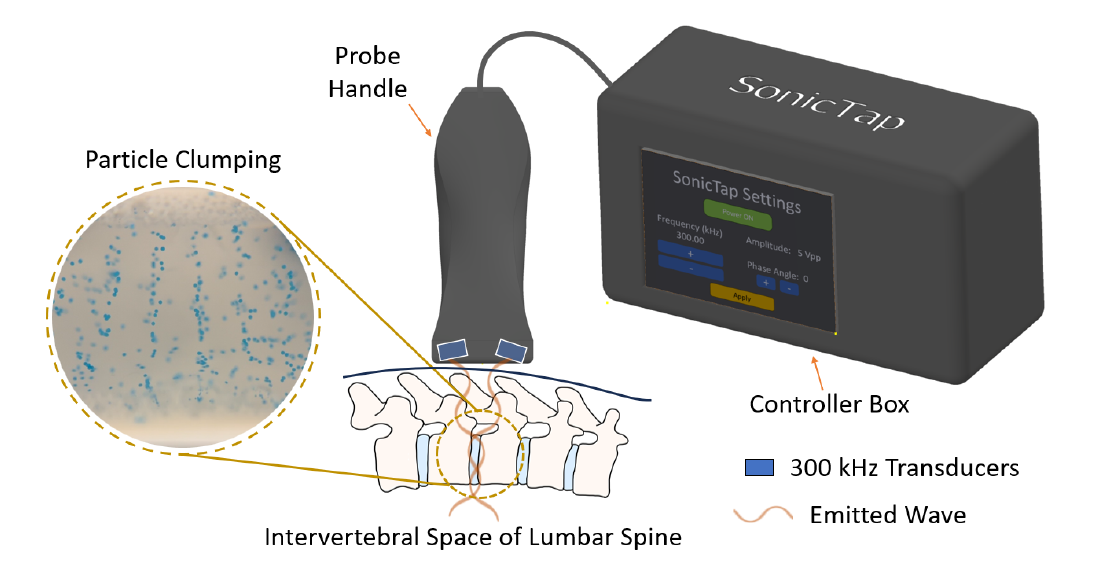
Meningitis is a bacterial or viral infection characterized by inflammation of the meninges, a complex system that serves as a protective barrier to the central nervous system. Adults with meningitis present with fever, sensitivity to light, headache with nausea, and stiff neck. Contrastingly, infants with meningitis exhibit suppressed symptoms, typically only presenting with fever and fussiness. Timely diagnosis and treatment of meningitis in infants is vital due to the potential long-term neurological deficits, such as learning disabilities and motor disorders. The current standard practice to diagnose meningitis involves performing a spinal tap, an invasive, painful, and emotionally distressing procedure to extract cerebrospinal fluid (CSF) using a needle inserted into the lumbar region. In normal physiology, CSF is primarily composed of glucose and other nutrients. Thus, the presence of immune cells in CSF is indicative of an infection. The overwhelming majority of spinal taps performed on infants produce a negative result, and instead, an upper respiratory virus or other more minor malady is present. Given the vulnerability of pediatric patients and the low incidence of meningitis (0.34 per 100,000 children), there is a need for a noninvasive method to indicate the potential presence of the disease in small babies (<1 year) to decrease the necessity of spinal taps, improve time to diagnosis, healthcare costs, and reduce the risk of complications. Our proposed solution involves using ultrasound technology to clump white blood cells (WBCs) in CSF by emitting sound waves at specified frequencies. Using a pre-existing medical ultrasound probe cutaneously placed over the lumbar region, the presence or absence of banding patterns formed from aggregated WBCs produced by our device can be visualized. If banding patterns are observed, it indicates that an infection may be present and that a spinal tap is required to make a diagnosis. Ultrasound is most widely used as a diagnostic imaging tool, but its ability to move particles in fluid has not been thoroughly validated or applied to detect infections. The novelty in our solution lies in the ability to aggregate cells in real-time in a clinical environment, using carefully selected and tunable frequencies. Once validated experimentally and clinically, this method can serve as a precursor for spinal tap procedures within hospital emergency rooms and outpatient settings, reducing the performance of invasive diagnostic interventions.
Talha Ahmad, Libby Bare, Jonah Carter, Wyatt Graddy, Roey Kuo
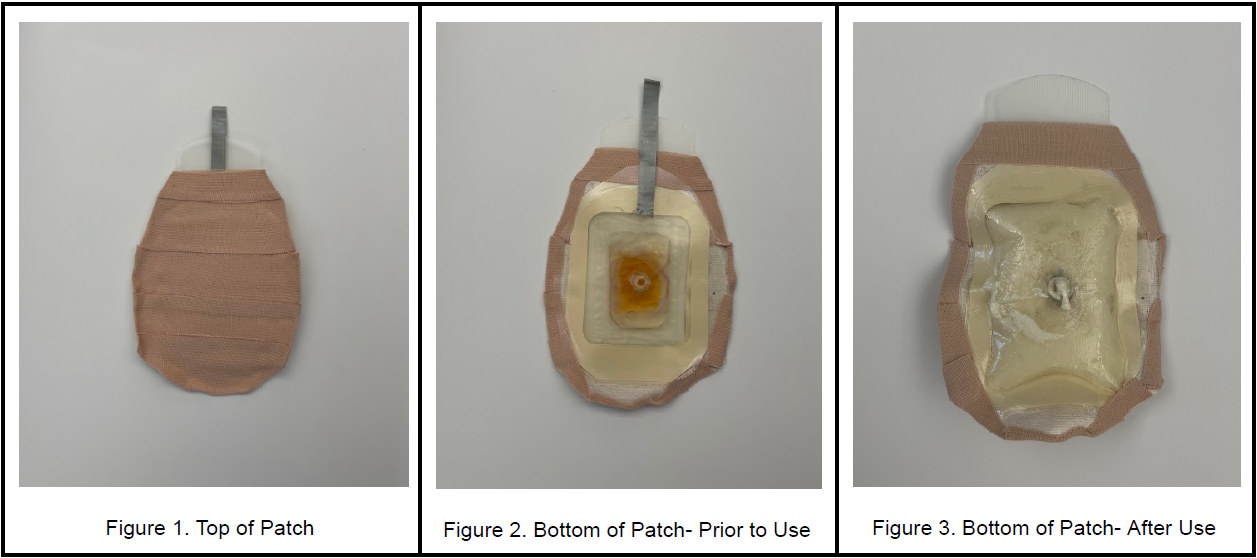
Since the Columbine shooting in 1999, there have been 783 shootings ranging from elementary schools to high schools. Yet, there is no in-class solution to prevent hemorrhagic blood loss in the abdominal region. Emergency medical services cannot be provided promptly in these shooting events, often leading to victims bleeding out. Therefore, a low-cost and easy-to-use solution is needed to increase trauma patient survivability in situations involving gunshot wounds to the thoracoabdominal region. The device consists of a patch with adhesive on one side, which is intended to adhere to the body of a victim of a gunshot wound. The patch contains two bags, an inner bag and an outer bag. The inner bag contains reagent A, and the outer bag contains reagent B. The inner bag is easy to pop, so that a child can pop it. When the inner bag is popped, the reagents will react to form a self-expanding polyurethane foam inside the outer bag. When the foam expands, there is a tab on the adhesive side of the patch which, once removed, allows the foam to exit the patch and enter the wound. It is important that the patch’s opening on the adhesive side be aligned with the victim’s gunshot wound for the foam to enter the victim’s body. Once inside the body, the foam will continue expanding due to the higher temperature inside the body. The foam will exert pressure inside the body to stem the bleeding inside the victim. This device is completely novel compared to the current solutions for stopping emergency bleeding, which include tourniquets and gauze. This patch is easy for children to use and injects biocompatible foam material into the patient’s body. Other similar innovations like ResQFoam or XStat are used for surgical or military settings, respectively. We will utilize ResQFoam’s foam technology, while entirely revamping the delivery method. No other device of its kind exists targeting the need to stop bleeding in school shootings. This device has the potential to greatly improve survivability for victims of school shootings by addressing the need to stop or curtail major abdominal or chest hemorrhages before emergency responders arrive and in an easy-to-use way that even children could administer.
Cameron Bresky, Megha Gupta, Kaniese Mack, Mary Maffei, and Mia Nair
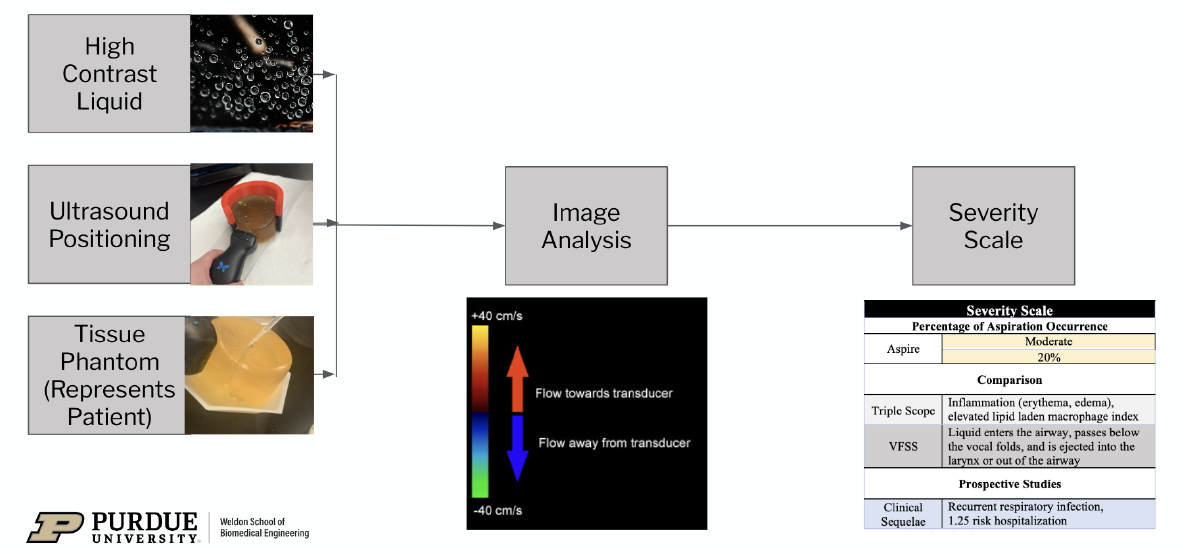
Aspiration is the accidental inhalation of foreign materials into the lower airway, leading to serious health complications if the severity is not quickly quantified and diagnosed. 35-40% of the pediatric population experience aspiration, with most patients too young to verbally communicate symptoms. The current methods of detecting aspiration, such as the Triple Scope procedure, which uses a flexible bronchoscope to assess the structure of the aerodigestive system while the patient is under general anesthesia, rely on invasive methods to observe the site of aspiration and lack the ability to quantitate the degree and severity. Therefore, there is a need for a non-invasive detection system to measure the level of aspiration in pediatrics that provides quantitative, accurate, and interpretable results. The team presents Aspire: a non-invasive, easy-to-interpret method that decreases procedural time and increases diagnostic accuracy and specificity compared to current technology. First, the pediatric patient swallows a thin, carbonated liquid chosen for its high contrast, clear visibility during ultrasound imaging, and increased likelihood of being aspirated. Patients are positioned in an adjustable, user friendly neck brace to ensure comfortable posture and correct ultrasound wand positioning. The ultrasound will utilize Color Doppler technology to obtain live footage of fluid passing through the esophagus. If liquid enters the trachea and is aspirated, image analysis software will detect the volume of liquid and output the percentage of aspiration occurrence via the use of a statistically trained predictive model. Lastly, a severity scale will provide clinical significance, grade the extent of aspiration, and justify future actions. Compared to the existing solutions on the market, Aspire proposes a non-invasive solution, which provides quantitative data regarding percentage of aspiration occurrence, and does not pose the additional health complications of general anesthesia. This proposed technology would provide the missing quantifiable indications needed for medical and/or surgical interventions for pediatrics.
McKenna Hillsdon-Smith, Graham Ragland, Jacob Hill, Elena Monrole, and Elyse Zurawski

The “Adaptive NERF Blaster Device for Users with VACTRL Syndrome” project was established in collaboration with the mother of a child with VACTRL Syndrome to create an adaptive NERF blaster device that can be effectively played with by a child with upper limb deformities. VACTRL syndrome is a health condition that can include any of the following complications – vertebral anomalies, anal atresia, cardiovascular anomalies, tracheoesophageal fistulas, esophageal atresia, renal anomalies, and limb defects. Specifically, the child inspiring the design of this device has radial deviation in both her left and right arms that significantly reduces her thumb function and fine motor skills, making it incredibly difficult to operate the triggers on a traditional NERF blaster product. This is not an uncommon problem, as VACTRL syndrome affects one in every 10,000 - 40,000 newborns, indicating a significant population of children with the potential for limb deformities that would limit their use of NERF blasters. NERF does not currently offer any modified blasters or adaptive accessories for existing blasters. Some independent retailers sell off-market variations of adaptively modified blasters or accessories, but the variety of options available is small and the cost tends to be anywhere between four and six times that of the equivalent unadapted device. The proposed design solution incorporates three modifications to a standard semiautomatic NERF Modulus Stryfe blaster: a hook-shaped front arm stabilizer, a rocker switch “rev” trigger, and a press button “blast” trigger. The trigger controls are moved to the outer surface of the blaster and the rocker switch and press button mechanisms simplify the motions required to activate the triggers. The hook-shaped front arm stabilizer allows users to aim without gripping the device with their dominant hand. These designs show innovative advancements to current NERF products that will make play fun and faster for all users, but especially those with upper limb deformities. The impact of this device is vast as it allows children with upper limb deformities to engage with their peers in similar play, thus positively impacting the child’s experience and mental health. By the end of the semester, the device will be a fully-functioning modified blaster that allows for accessible play by someone with upper limb deformities due to VACTRL syndrome.
Yousef Altayeb, Nicole Ashta, Emilie Chadwell, Jordan Lyles, and Saaniya Rupani
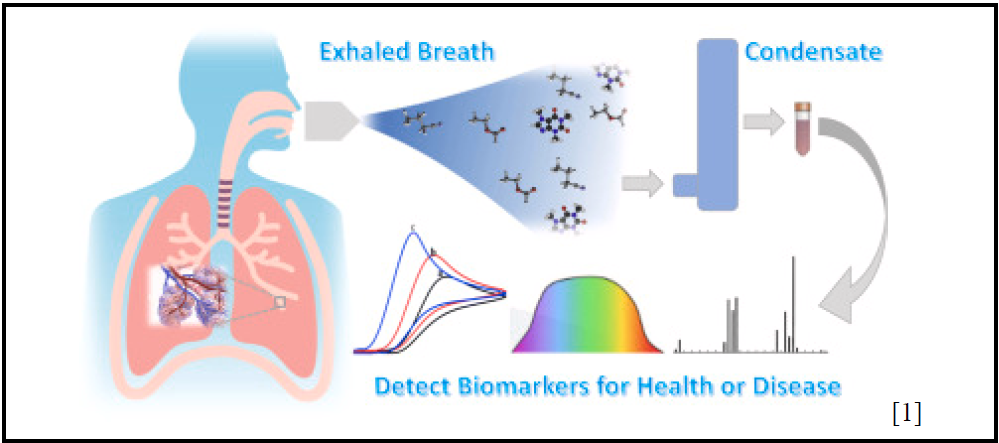
Volatile Organic Compound (VOC) breath analytics represents a cutting-edge frontier in non-invasive diagnostics, offering a wealth of potential for revolutionizing healthcare assessments. There is a clear need for enhanced breath analytic devices that can effectively address sensitivity, selectivity, and accessibility issues in VOC monitoring devices, which are crucial for advancing solutions in disease monitoring. In particular, given the lack of commercially available VOC sensors, our primary emphasis will be on developing accessories to ketone sensors to improve diabetes monitoring. Our objective is to develop noninvasive, inexpensive, and accurate accessories to breath analytic devices for diabetes patients. Conventional ketone measurement methods often entail uncomfortable and expensive blood tests, which can be avoided with our solution. A crucial refinement lies in augmenting the sample size for breath analytic tools, thereby heightening device sensitivity – this relationship between sample volume and sensitivity has been substantiated in research, affirming that larger sample sizes yield enhanced analytical precision. We will assess sensitivity by comparing ketone measurements in subjects before and after applying our accessory, yielding quantifiable evidence of the extent of improvement. Additionally, we will incorporate a specialized filter within the accessory device to heighten selectivity in ketone detection amidst a mixture of VOCs. This enhancement in compound collection efficiency will be directly gauged on the device. Comparative testing – both with and without our accessory – will be conducted to evaluate selectivity improvement. Finally, we will ensure that the device is designed within our budget, guaranteeing accessibility for a broad spectrum of diabetes patients. There is a distinct contrast in the capabilities of the current commercially available devices for diabetes monitoring and emerging technology in the field of breath analytics. The standard blood glucose monitor and continuous blood glucose monitor require invasive blood samples for analysis, while emerging devices only require breath samples for analysis. Blood glucose measurements are the gold-standard of care because the equipment is often covered by insurance, and it provides a real-time reading of blood glucose levels. For some, however, these tests can be painful and time-consuming, highlighting a gap in diabetes management. As such, monitoring a patient’s acetone level in a noninvasive manner through breath analysis provides patients with the opportunity to quickly and easily check their blood glucose from their acetone levels – this allows patients to conserve the use of blood glucose strips by testing only when out-of-range values are detected, rather than monitoring blood levels throughout the day. Expanding the sample size and refining VOC selectivity has the potential to elevate the precision of existing portable designs, presenting a less intrusive technology for routine monitoring. Such an advancement could mark a significant milestone in VOC testing, profoundly influencing the field of medicine through enhanced diagnostic capabilities.
Aavya Srivastava, Ari Aghevli, Andrew Frels, and Allison Lao
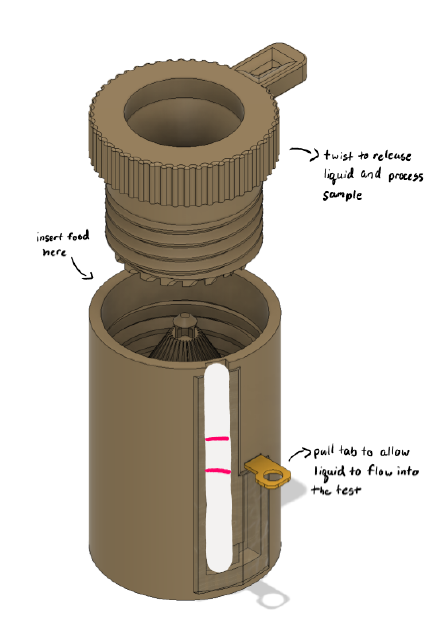
Around 33 million people in the United States suffer from gluten-related disorders, of which 3.3 million suffer from celiac disease. For these people, the avoidance of gluten is necessary in daily life, so ensuring that food and cosmetics are allergen-safe would increase their quality of life. Currently, there is no easy-to-use and portable method available to determine if foods and cosmetics are allergen-free. The solutions that do exist for this problem are either electronic in nature and require multiple components, or they can only be properly used in a laboratory setting. This highlights the need for a solution tailored to the adult gluten-sensitive demographic that is easy-to-use, portable, and delivers prompt results. Our product, Allaware, is a novel approach to the allergen testing market. Unlike other products, Allaware includes a processing component that is integrated into the device to process food or cosmetic samples, eliminating the need for users to break up the sample themselves. The user inputs the food at the top, and the device grinds the sample mechanically with just a few twists. This helps to increase the safety of allergen testing because the user does not have to directly touch the sample or use utensils to break the sample into tiny pieces. The lateral flow testing component is connected to the processing component, eliminating the need for users to utilize multiple components to use the device. The entire device is disposable to remove the need for rigorous cleaning following a positive test result. Our product provides a substantial impact on medicine based on several factors. From a health perspective, our device can prevent patients from having allergic reactions in the first place, ensuring they do not go into anaphylactic shock. The Allaware testing system can also benefit patients from an economical perspective, given they may lose working hours if sick or have to use an EpiPen, which can cost over 650 USD for a one-time-use device. Overall, this product is time-efficient and improves the consumer’s quality of life.
Karsyn Kazyak, Anna Lacerda, Kaycee Baird, and Grace Salyers
In the United States each year, approximately 150,000 patients have part of their lower limb amputated. Diabetes and vascular disease are the top two causes of lower limb amputation, and diabetes rates are projected to increase by almost 120% before 2030. Prostheses for transtibial (lower limb) amputations are made to fit tightly, precisely, and specifically to each person and do not account for day-to-day shrinking and swelling of the residual limb. For an adult, below-knee amputee utilizing a prosthesis, there is a need for an adjustable socket that can accommodate the daily fluctuations of volume in the residual limb. The solution should be manually adjustable for its wearer and should adapt to both swelling and shrinking. Our solution to this problem consists of a three part system incorporating a gel bead base, a mechanically adjusted air bladder liner, and an adjustable socket cohesively combating residual limb volume change. The gel bead base will consist of a packet of uniformly distributed gels to provide constant pressure to the base of the limb. The air bladder liner will be constructed as an air bladder grid system accounting for common points of volume change to allow for manual modifications throughout the day by the push of a button. The final component will be the outer shell and the socket that secure the system to the limb and allow for additional volume adjustments. The combination of these methods brings a fresh approach to prosthetic socket fit, combatting the rigidity of current devices. This approach is also financially accessible, as mechanical approaches are more often insured than electrical. Overall, this design and innovation will bring a novel solution to transtibial amputees to address their residual limb volume change with comfort, ease and adjustability.
Joshua Brody, Emma Davis, Giancarlo Mella, Caitlin Savage, and Nikhil Varanasi
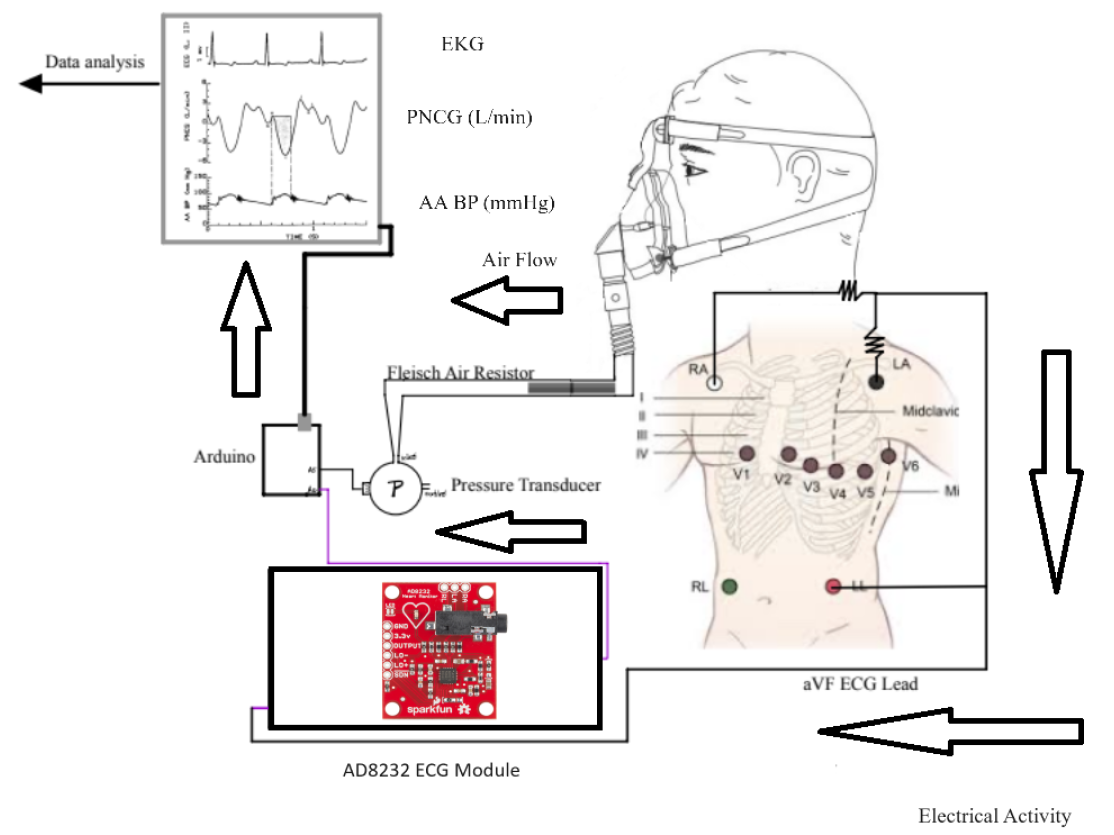
A successful design will be capable of measuring stroke volume (SV) and heart rate (HR) to mathematically calculate cardiac output. This is done by utilizing the cardiogenic airflow oscillations that result from cyclic changes in thoracic blood volume during systole(ventricular ejection) and diastole(ventricular filling). A recording of these airflow oscillations is called a pneumocardiogram (PNCG). The patients will exhale into an airtight mask and the air will flow through a Fleisch air resistor in the device. The resistor generates a pressure difference, which is measured by a transducer acting as a pneumotachograph. Flow rate is determined by performing the quotient of differential pressure and resistance. The purpose of collecting the flow rate of the exhale is to capture the PNCG signal. Integrating the segment of the PNCG signal that corresponds to left ventricular ejection time (LVET) allows SV to be captured from the PNCG signal. To identify LVET, the patient’s EKG signal must simultaneously be collected while their exhalation is being recorded. The EKG will also provide the heart rate, which is needed to calculate CO. It is imperative that the EKG and PNCG data are collected at the same time to maintain consistency in the calculation. The RC (time constant) value represents the low-pass filtering effect created by airway resistance and lung-chest compliance. This metric is necessary in determining final stroke volume. This design provides a low-cost, non-invasive option that utilizes the genesis of a pneumocardiogram and simple mathematical techniques to determine cardiac output and stroke volume. There is not a solution currently on the market that utilizes a pneumocardiogram signal in awake, non-anesthetized patients to assess heart function. This design will allow for the determination of a patient’s heart function by simple measurements of intrathoracic pressure during ventilation with an adapted CPAP mask. The impact of this technology could alter how patient heart function and activity are routinely monitored within non-critically ill patients and high-performance athletes. This solution would provide patients with a non-invasive alternative to common procedures, present a fiscally justifiable option to reach more customer segments, and is optimally designed for ease of use by clinicians and practitioners.
Alex Bergendorf, Jett Stad, Priyadarshini Subramaniam, Sveni Thalor, and Molly Tredway
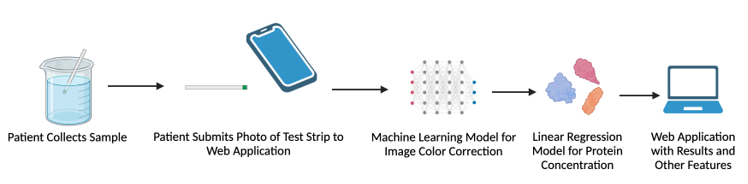
Approximately 37 million people in the United States are living with chronic kidney disease (CKD) . CKD is classified into five stages of varying severity, ranging from mild to kidney failure. Patients are assigned stages based on glomerular filtration rate (GFR) and albuminuria. GFR is difficult to measure, and is typically estimated based on serum creatinine levels. Albuminuria can be monitored through the amount of the protein albumin in urine. Thus, CKD is often diagnosed and monitored through albuminuria. Albumin can be detected through urinalysis, typically using a dip-stick test. Urinalysis requires a trip to a lab or doctor’s office, costing the patient anywhere between $30-247. At home urine protein testing is possible, however, nephrologists have identified that patients struggle to interpret at home urine protein test strips. Disease management is based upon the stage of CKD, and appropriate treatment slows progression, reduces the risk of cardiovascular disease, and improves patient survival and quality of life. Appropriate stage classification is vital in ensuring the patient is receiving the correct treatment. However, patients with CKD typically follow up with their doctor every 1 to 6 months, potentially creating large gaps in disease monitoring. Thus, a way to monitor CKD severity in patients at home that saves time, money, and healthcare resources is needed. The goal of this project is to develop a smartphone application that uses a machine learning model to color-correct pictures of urinalysis strips and provide accurate colorimetric readings of urine protein. Using commercially-available urine strips allows for convenient at-home monitoring, while still providing the accuracy of tests that can currently only be done with a uACR test in a medical office. In addition, since the solution consists only of affordable urine strips (~$15 for 30 strips) and a web application, it will be accessible for all CKD patients. This allows us to bridge the gap for medical disparities, since CKD patients are disproportionately minorities and from socioeconomically disadvantaged backgrounds. The novel aspect of this solution is ultimately that it is focused on CKD monitoring. Although several other colorimetric-based diagnostic apps exist both commercially and within literature, none are designed specifically for measuring protein to monitor CKD. The machine learning color-correction algorithm is also novel and not available in other apps. This provides a new level of accuracy not currently possible for commercial urine test strips. Although existing accurate methods are available for CKD, such as in-clinic uACR testing, none are as affordable and convenient as at-home test strips. The rapid nature of the test strips also allows for point-of-care monitoring. This combination of accuracy, accessibility, and convenience is not currently available in any existing or emerging solution.
Bryce Johnston, Vishnu Kamagere, Christian Peterson, Chenlong Li, and Maverick Broviak
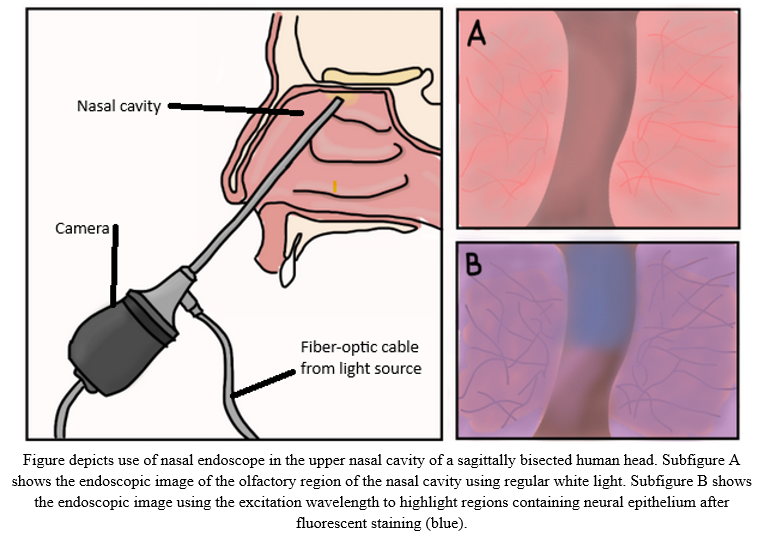
The clinical need of our project is to produce an easy to use and reliable modification for current nasal endoscopes that allow for accurate visualization and detection of neural epithelium tissue, present in the upper olfactory regions of the nasal cavity, after fluorescent staining to improve the quality of life for nasal endoscopy patients. This would result in reducing the need for biopsies for tissue identification, decreasing diagnostic time, and improving precision in surgical procedures; thus, lowering healthcare costs and reducing the need for intrusive tissue damage. The solution will consist of four parts: the fluorescent dye, light source, light focusing system, and the image processing component. The most effective way to accomplish the fluorescent staining of only neural epithelium is with immunofluorescence, where an antibody conjugated to a fluorophore binds only to proteins found on the membrane of neural epithelial cells. The light module consists of two key components. First, a narrow-band light source generates a peak wavelength at which the fluorophore maximally fluoresces, providing visualization of the neural tissue. Secondly, this light goes through a set of lenses and a compound parabolic concentrator to maximally focus the source into the fiber-optic cable of the endoscope. Images from the endoscope will be input into the image processing software package which will use the fluorescence tissue emissions resulting from the narrow-band light excitation to determine the location of neural epithelium against other tissues. The design innovates within the field of nasal endoscopy by providing a way to see the same tissue in a new light - the new light being what is emitted from the fluorophore after excitation by the light source. Standard NBI (narrow-band imaging) is specifically tuned to make blood vessels more visible, but with this innovation, the same principles can be applied to visualize much more difficult-to-find structures of interest, such as neural epithelium. Locating the neural epithelium should prove very useful to the medical field, as it will allow for progression tracking of Alzheimer’s. Our improved endoscope design aims to transform nasal diagnostics and therapies by labeling neural epithelium with a fluorescent marker. With the ability to precisely detect and distinguish neural epithelium in real-time, clinicians should expect a significant reduction in the need for invasive biopsies, resulting in little discomfort and dangers for patients. Furthermore, by substantially shortening diagnosis times, patients benefit from accelerated treatment programs, potentially leading to better outcomes. Lastly, the increased precision provided by this breakthrough will allow surgeons to carry out surgeries with better confidence and accuracy, reducing potential problems and post-operative concerns. These developments, taken together, have the ability to significantly improve nasal endoscopy patients’ experience and quality of life.
Nolan Shaw, Marlee Shepherd, Reagan Smiley, Kaushik Subramanian, and Benji Wilkins
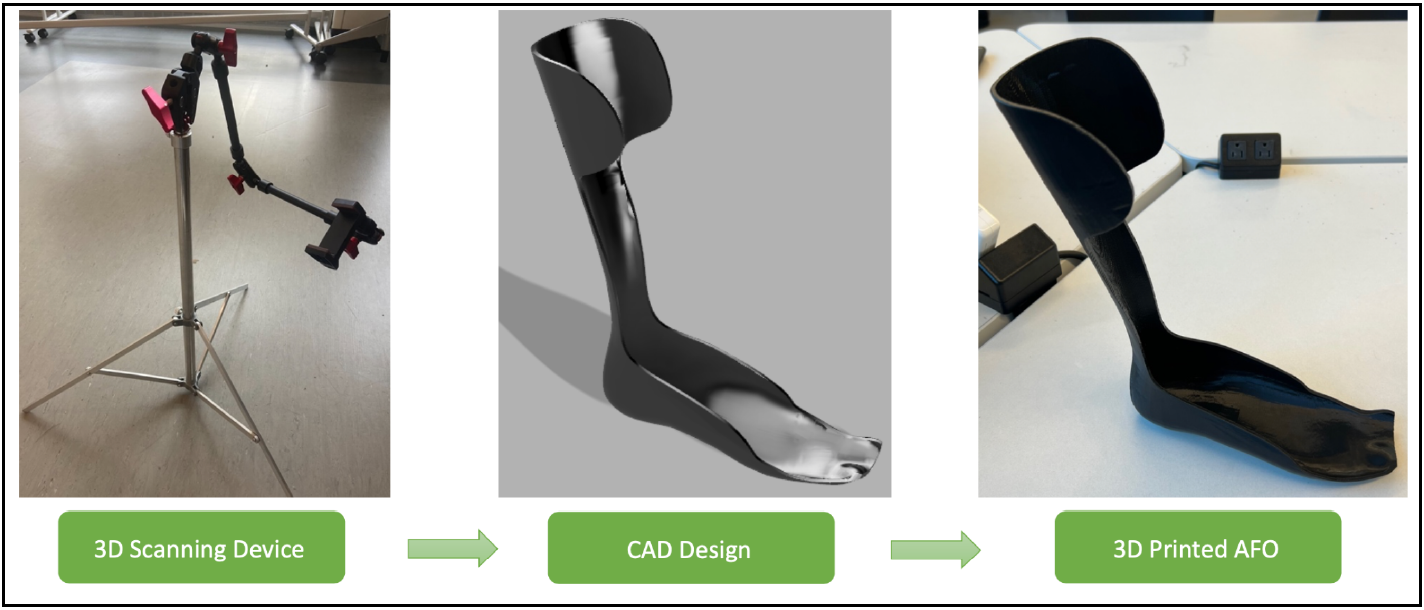
The ankle and lower leg are a critical part of the body, enabling movement, balance, and stability. Neurological, musculoskeletal, degenerative, congenital, and developmental conditions as well as acquired impairments and injuries all have the possibility of severely altering these functions in the lower limbs. Some examples that are being considered include cerebral palsy, spina bifida, muscular dystrophy, and multiple sclerosis. To address the common negative outcomes of these diseases, the team is designing ankle-foot orthotics (AFOs) to restore gait and normal use of the lower limb. Like most of medicine, technology is becoming increasingly personalized, and the goal of this project is to create custom, easily accessible orthotics for pediatric patients. Each disorder has variability and unique anatomical differences that the AFO design will need to address through providing extra support for certain areas of the foot. The custom AFO will provide increased mobility and support, control alignment, prevent further deformity, and assist with rehabilitation. The team is partnered with RoverLabs, a medical technology company based in Tanzania, that is focused on prosthetics and orthotics. The project will include a 3D scanning rig, as shown on the left in Figure 1, and a system to scan the affected limb and print the appropriate rigid AFO, shown on the right of the figure. It will need to be affordable, durable, comfortable, and effective. Currently, scanning and manufacturing techniques vary in use depending on physician and lack detailed specificity from patient to patient. Therefore, the design will improve upon this to produce an AFO that truly conforms to the shape of the user. The AFOs must be time- and cost-effective to ensure access to all who need it. This is a crucial change that could facilitate the increased reach of AFOs across Tanzania and the developing world at large.
Kate Christensen, Emma Goebel, Meghan Gron, Mae Kinst, and Ranya Pendyala
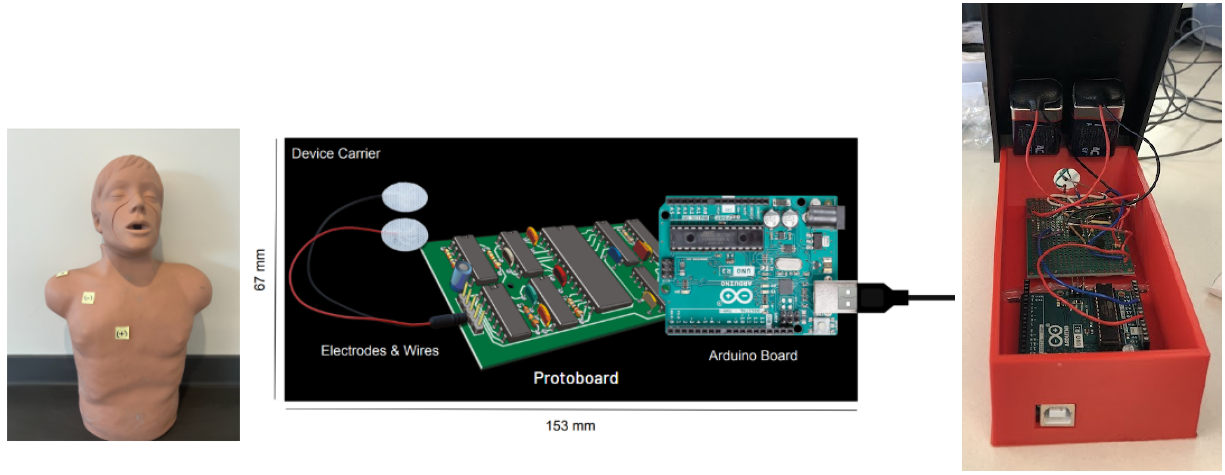
Pectoralis muscle (PM) injury typically occurs due to excessive tension from weightlifting exercises or direct trauma [1]. Patients with PM injuries exhibit limited, painful range of motion, localized swelling and ecchymosis, and tenderness. Currently, magnetic resonance imaging (MRI) is the standard diagnostic tool for these injuries and can cost between $500-$7900. Imaging expenses may deter patients from seeking medical attention, as 50% of PM injuries go misdiagnosed. Misdiagnosis can lead to further muscle injury, such as tears, which require more invasive treatment options and longer recovery times. Therefore, a low-cost, non-invasive, and user-friendly diagnostic tool is needed to decrease misdiagnosis and prevent further injury. Opportunity for an alternative method for pectoral muscle strain diagnosis is present in electromyography (EMG) signals from muscle activation. Slight contraction of the muscle results in some electrical activity, which increases as the muscle contracts more intensely. An EMG can diagnose injuries or diseases that affect muscles and determine the presence, location and extent of these injuries. Thus, our team is designing a solution that utilizes EMG sensors connected to a protoboard, which filters noise and amplifies the output pectoral muscle signal for display. Patients will be asked to perform exercises to activate the pectoral muscle for further signal analysis. When compared to the speed and amplitude of a baseline (healthy muscle) signal, muscle signals indicating strain or complete tear should be apparent. This solution is significantly less expensive than current methods, non-invasive, and allows users more autonomy in managing and tracking their own injuries. The instruction manual given with the device allows athletic departments and athletes to use the device and interpret the results without the professional assistance of a doctor or engineer. Ultimately, the device is a cost-effective, non-invasive solution for diagnosing pectoral muscle strain to prevent further injury and reduce incidence of misdiagnoses.
Claire Giles, Alex Memmer, Naomi Mueller, and Hayden Plack
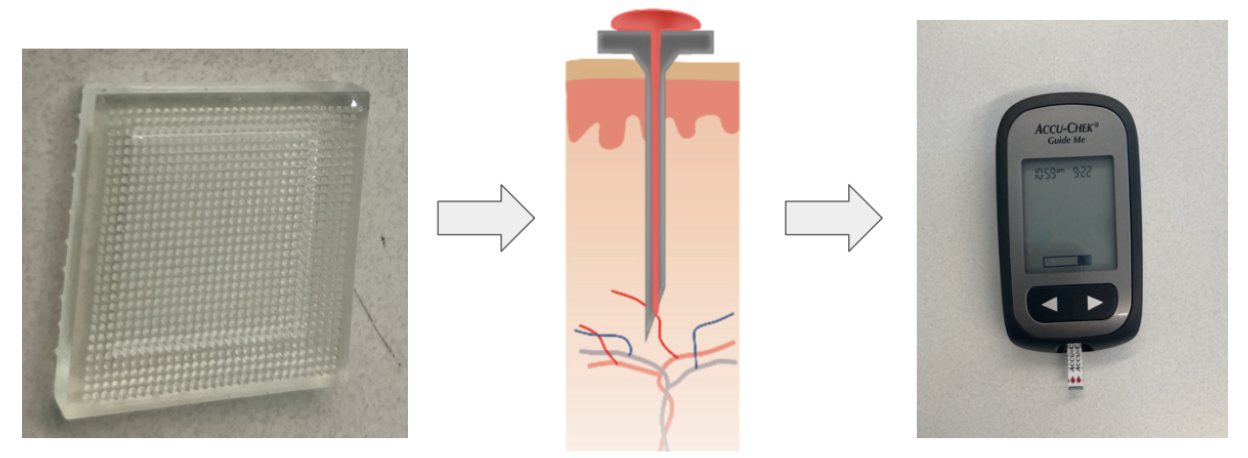
There is a need to measure blood glucose levels that is less painful than the existing lancet and test strip method while being accurate, low cost, and easy for all diabetes patients to use. The solution will be a patch containing one side of hydrogel microneedles and a bandage backing. The user will apply the patch to the skin and allow the microneedles to collect a 1 microliter sample of blood via capillary action. Microneedles have been used for blood collection clinically, but have yet to be integrated into blood glucose test strips, which removes the need for use of painful lancets. This device will create a less painful way of collecting a blood sample for blood glucose testing to create a more user-friendly experience for diabetic patients worldwide. This could potentially increase user testing frequency and eliminate calluses produced from repeated lancet piercing which inhibits the ability to accurately collect a blood sample.
Chloe Kim, Olivia Loesch, Chloe Matasovsky, Elizabeth Murphy, and Arden Shen
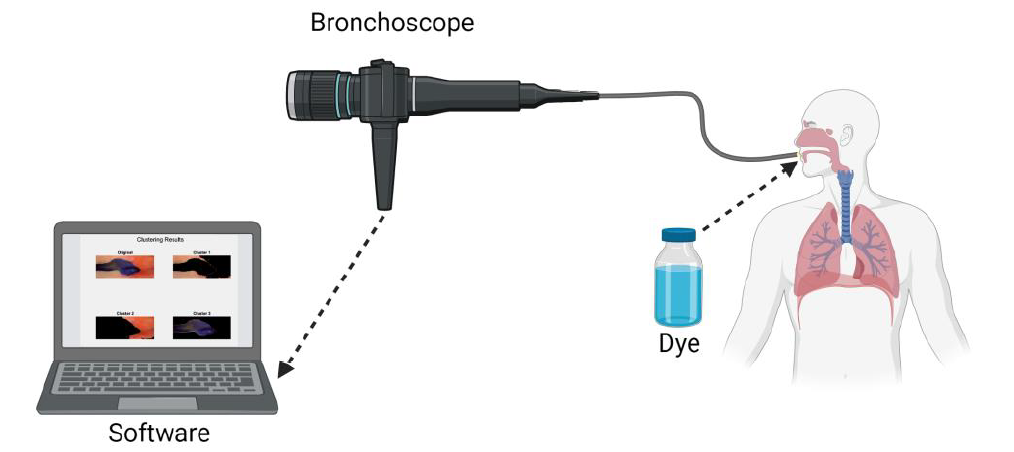
Chronic micro-aspiration can affect up to 40% of pediatric patients and lead to clinically significant sequelae, including recurrent hospitalizations for respiratory infections. Presently, there is no gold standard test to diagnose or quantitate pediatric aspiration. Current methods such as videofluoroscopic swallow study and biomarkers lack the ability to quantify the degree and extent of aspiration. Our proposed technology would provide the missing quantifiable indications needed for medical and/or surgical interventions. Our project aims to create a bronchoscopic system capable of visualizing and quantitating aspiration. We propose to identify a dye that can be given orally, in a safe volume, before a bronchoscope procedures, with the intent to stain the airway with the aspirated dye. Then, images or videos obtained from the bronchoscope procedure will be passed to our novel image processing algorithm to identify the stained areas and quantify the amount of dye stain. This quantification metric will be provided to healthcare professionals for severity assessment. Our new technology would provide a revolutionary method of quantifying severity and extent of chronic micro-aspiration, and subsequently, allow further investigations into the clinical course and response to differing interventions. This information could eventually be used to establish medical management guidelines for pediatric aspiration patients. A certain degree of aspiration detected on our new technology may help indicate conservative versus surgical intervention (such as gastrostomy, fundoplication, salivary duct ligation, etc.).
Sean Devaney, Jessica Joslyn, Amy Lederer, Haley Rickey, and Kaitlin Zeltwanger
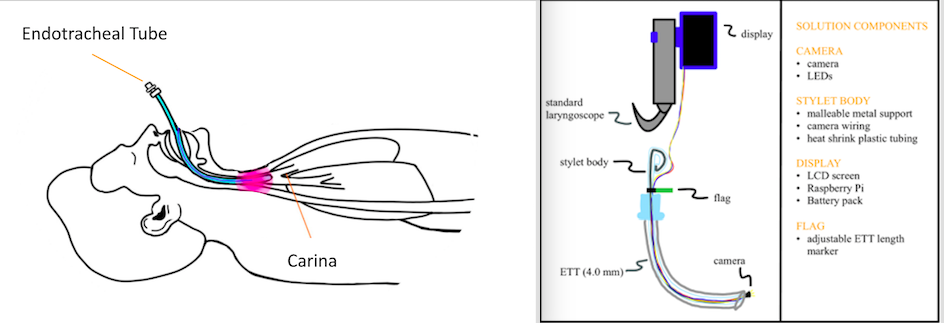
Up to 85% of preterm infants require intubation and 3-4 attempts are often needed to successfully intubate. Malposition is common and can lead to adverse effects in neonatal patients. Currently, placement confirmation of endotracheal tubes (ETT) requires chest x-rays, which provide delayed results, can expose neonates to large amounts of radiation if multiple attempts are necessary, and are costly. To address these problems with ETT placement in neonatal patients, we are proposing an innovative solution that will provide immediate confirmation of correct placement at approximately 0.5-1 cm above the carina and limit radiation exposure to less than that of an x-ray (0.02 mSv). Our novel solution is an ETT stylet with a camera at the distal end to allow for real-time visualization of the neonate's anatomy, specifically the carina, during intubation to ensure correct ETT placement. With our design, healthcare providers will be able to not only visualize the carina via live feed but also have a way to confirm that they are in the correct position without having to do another procedure or use another device. The number of trials for correct placement will be decreased to 1 for most patients, therefore decreasing the frequency of x-rays. This real-time placement confirmation of ETTs can decrease the frequency of several adverse effects, including bradycardia, desaturation, right main stem placement, and airway injury for the large group of neonatal patients as well as save time for caregivers in the extremely busy setting of the NICU.
Jordan VanArsdale, Kaitlyn Reagen, Sasha Gonzalez, Logan Garvey, and Sydney Loudermilk
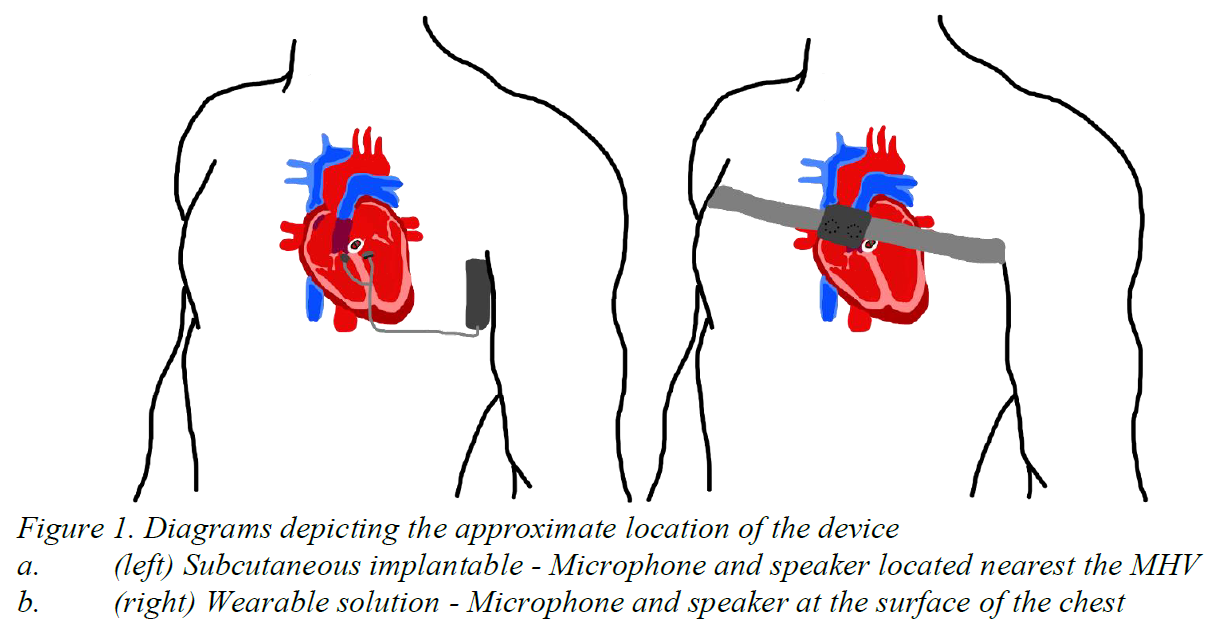
Around 280,000 heart valves are replaced annually, worldwide due to congenital heart disease and valve deformities [1]. Mechanical heart valves (MHVs) make-up half of these procedures [1]. While life sustaining, 68% of MHV patients express annoyance, 56% experience sleep disturbances, 36% develop concentration issues, and 28% develop social embarrassment due to the constant clicking noise generated by MHVs [2]. A survey conducted in 2017, revealed that 28% of patients were willing to undergo open heart surgery again, just to eliminate the noise [3]. As of 2023, there are no existing technologies created to induce lasting and effective mechanical heart valve noise reduction. Our solution will take the form as either a subcutaneous implantable (Figure 1a) or wearable device (Figure 1b) that produces an antiphase waveform to actively attenuate the MHV clicking noise. The device will be composed of a microphone, speaker, and circuit inside of a biocompatible shell. Ultimately, our solution will produce lasting and effective active noise reduction to alleviate patient sleep and psychological disturbances.
Luke Browning, Nick Most, Sunita Nhemafuki, and Toby Miller
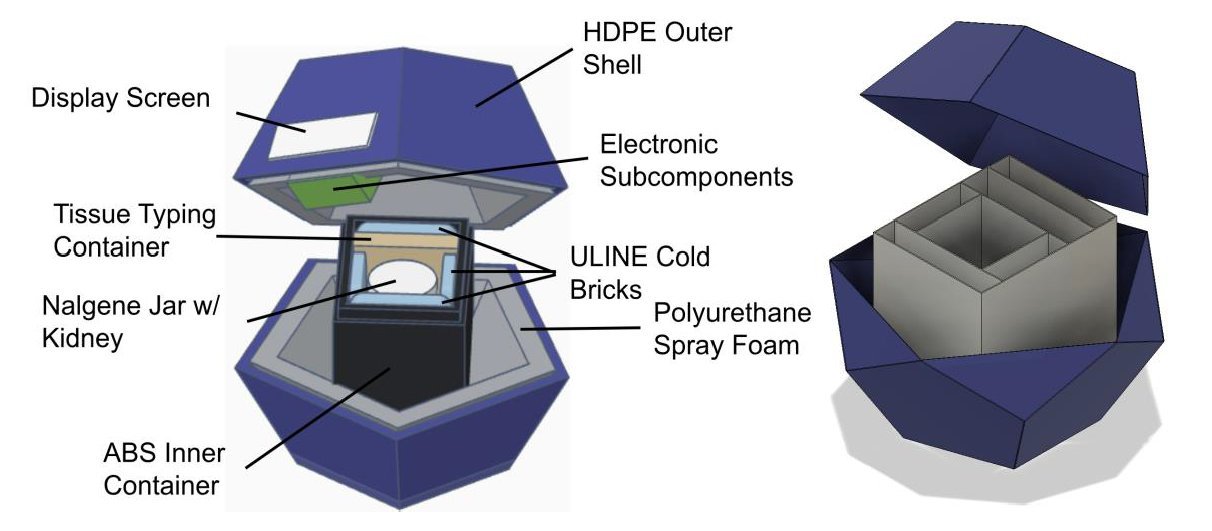
In the United States, over 5,000 people die annually while on the kidney transplant waitlist, sometimes with wait times as long as 8 years. One of the presenting issues is that surgeons will not transplant a pair of kidneys if there are any questions about viability, which causes 20% of pairs of donated kidneys (roughly 7,500 kidneys) to be thrown out every year. Kidneys outside the body are functional only for 24 - 36 hours when stored properly within the temperature range of 4 - 8 °C, and sometimes the kidneys being transported are left at gas stations by couriers handling the transport or misplaced inside the transplant center, making it difficult to locate. Temperature and location of the kidney during transport are two key factors in determining transplant viability; without this information, surgeons are unable to make confident assertions about viability. The current market of kidney transportation coolers either does not offer these temperature and location tracking capabilities or is far too expensive for normal use; therefore, an affordable method of kidney transportation is needed for surgeons to monitor and record information about temperature and location while kidneys are being transported to their transplantation site. Our solution must be able to maintain viable kidney temperature (4 - 8 °C) for about 20 hours while also providing real-time temperature and location data to the appropriate medical and organ transportation staff on a website. The cooler consists of two main subcomponents: the outer and inner containers. The outer container is in the shape of a dodecahedron, with a hard outer shell and closed cell insulation foam on the inner layer containing a 3D-printed box that houses the kidney, cold packs, and blood vials. The electrical components are housed near one of the walls of the outer container and connected to an LCD screen visible to the user that displays real-time temperature, ensuring the functionality of the circuit. All temperature and location data will be recorded and relayed to a website platform, accessible only by the appropriate stakeholders. Our design presents a unique solution to this healthcare problem by delivering advanced tracking capabilities at an effective cost. We expect our device to take over the current market of organ transportation devices and allow surgeons to make better-informed decisions about kidney viability, leading to fewer kidneys being discarded each year and more lives being saved as a result.
Josh Sexton, Shaiv Mehra, Andrew Gangstad, and Tommy Mullin
Christopher Chang, Zane Barker, Winston Ngo, and Joe Pierzakowski
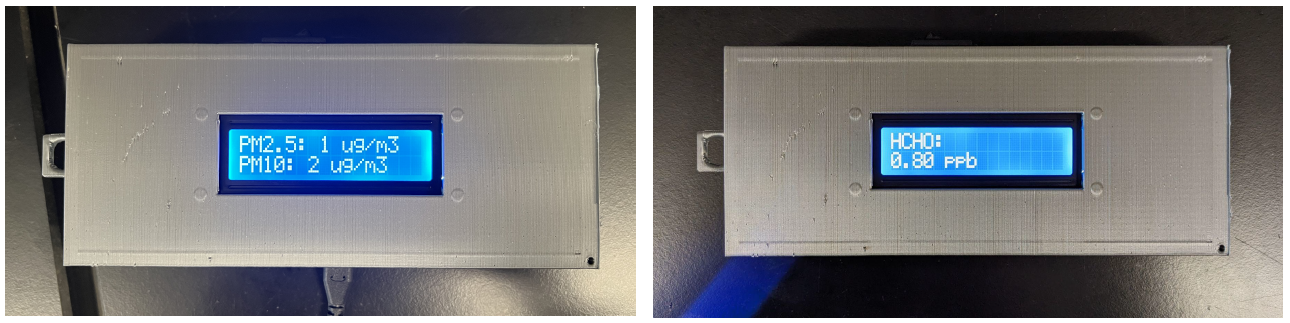
34 million people in the US suffer from some form of chronic respiratory disease such as asthma or COPD. These individuals are more susceptible to adverse effects such as coughing or wheezing due to moderate to high levels of air pollution. A solution to address portable, personal air quality monitoring would enable both these individuals and others to take preventative measures to protect their health. This device measures the concentration of PM, with diameters of 2.5 and 10 microns, and formaldehyde in the air. When dangerous levels of formaldehyde and PM are detected in the device’s surroundings, the device will vibrate and flash a warning message on the display so the user can seek a safer environment. The device is specifically designed to measure formaldehyde and PM. Other devices are more broad with their VOC detection and do not specifically address formaldehyde. The code for all components is open source, so anyone can understand how the device works and adjust it to fit their needs. Overall, the device is cost effective and easy to manufacture. By providing the formaldehyde and PM concentrations, it allows people to determine if their health is jeopardized in different environments. Additionally, this will allow users to make changes in their homes and businesses to lower and control dangerous formaldehyde and PM concentrations. As a result, this will minimize the incidence of events of severe respiratory distress. In turn, this will limit the amount of money users are spending on medications and treatments for respiratory issues created by their environment.
Grace Stephenson, Mackensie Hall, Leia Schiltz, and Maggie Hanlon
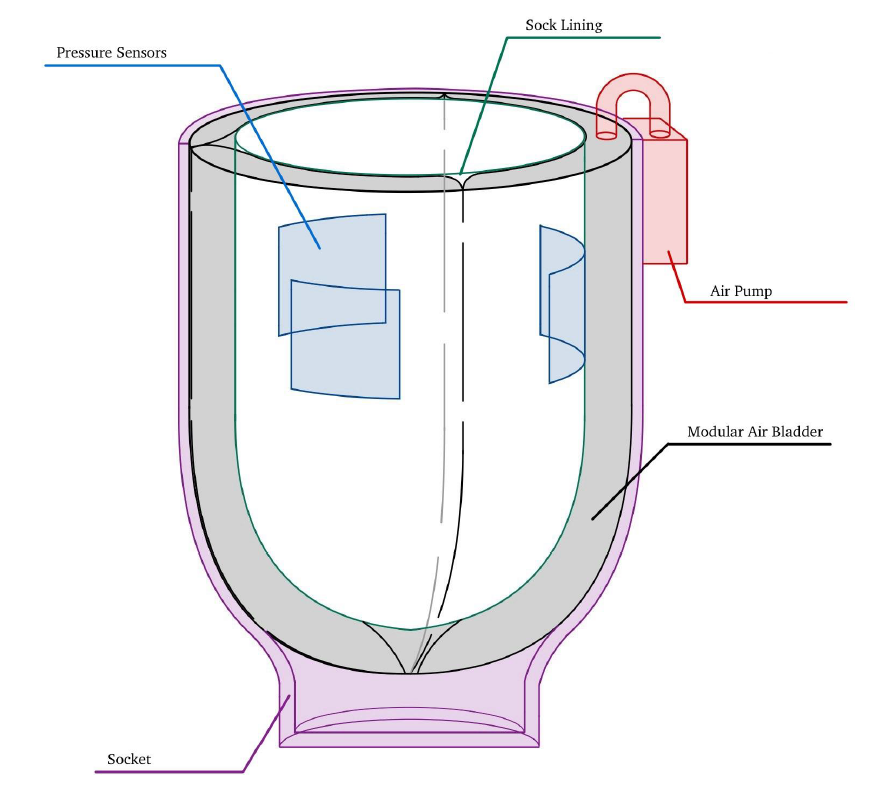
One hundred fifty thousand lower-limb amputations occur each year in the US; many of these amputations occur due to complications associated with diabetes or cardiovascular disease, and 51%-92% of lower-limb amputees have either diabetes or peripheral arterial disease. During the first two years post-surgery, the residual limb volume changes drastically, which can cause the patient’s prosthetic socket to fit improperly, leading to pain and discomfort. Those with other comorbidities are highly susceptible to more significant volume changes in the lower limbs. Current devices available for adjusting prosthetic fit address small regulated volume changes (up to 4 cm in diameter) and are not covered by insurance. There is a need for a cost-efficient prosthetic device to address discomfort caused by significant volume changes (upwards of 6 cm in diameter) for post-transtibial amputees. ResiComfort provides a low-cost solution to this clinical problem by automatically adjusting the circumference around the residual limb. The most important features of this device include automation, cost-efficiency, its small size and lightweight design, and the fact that it is easy to apply to existing prosthetic sockets. ResiComfort features an air bladder system connected to pressure systems situated between the bladder and the residual limb; these sensors will measure pressure values on the limb and send feedback to an air pump on the outside of the socket. The air pump should either inflate or deflate the bladder based on the pressure values received, preventing overtightening and under-tightening of the limb. The solution should create a uniform circumferential pressure along the residual limb, automatically maintain a safe and comfortable pressure range (for patients with lower health literacy), and be detachable from the socket, allowing it to be used by patients with different socket types. Although some current solutions on the market use air bladders to adjust the prosthetic fit, they are manually adjusted and ultimately are a high-cost investment. Existing solutions do not account for sizable residual limb volume changes during the first two years after amputation; instead, they account for more minor, daily changes in the residual limb volume. Our solution fills the gap in the current market by providing a cost-effective, automated method of addressing large residual limb volume changes for amputees in the first 1.5-2 years after an amputation. This device is anticipated to significantly reduce the patient costs associated with regular prosthetic refittings during the first two years post-operation. This device will also provide increased comfort for patients with significant residual limb volume changes in the first two years after operation. This will go a long way in improving the patient's gait reeducation, and mental and physical health as they begin to navigate life with a prosthetic.
Marge Aaron, Chloe Rouse, Braden Stock, Caroline Rakowski, and Wilson Berry
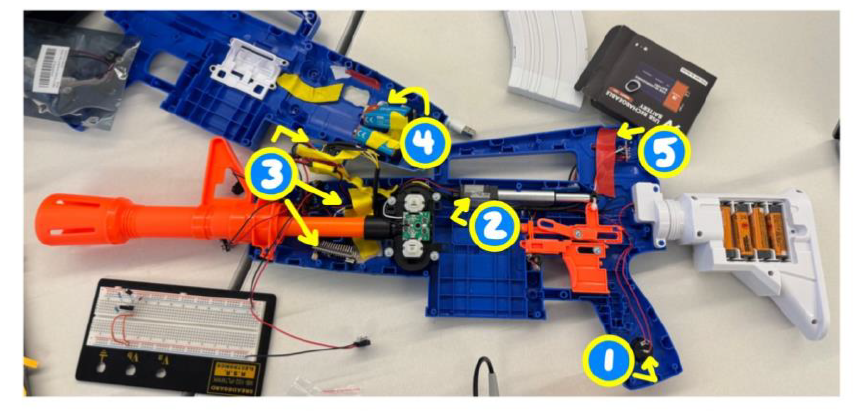

VACTRL syndrome is a condition that is characterized by multiple congenital malformations including vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal anomalies, and limb abnormalities. Therefore, individuals with VACTRL suffer from mobility issues due to absence or malformed bones, which limits the person’s ability to utilize complicated devices including NERF blasters. For these users and our client, this results in feelings of isolation and exclusion when others are operating NERF blasters, and this is indicative of a need to develop an assistive device for the user that allows the user to autonomously operate NERF blasters in order to eradicate exclusion from group setting and improve well-being. In conditions like VACTRL, every child has unique symptoms that disrupt their normal movement. Criteria such as strength, limb usage, or skeletal structure are distinctive for each patient, therefore one solution may not be applicable for every child. This lack of specificity creates an innovative market for our product to operate in by designing user specific adaptable toys. Our proposed solution is designed specifically for one user and incorporates switch adaptations that include a button that bypasses the need to conventionally pull the trigger in order to make the blaster easier to operate and fire. Additionally, the solution includes a linear actuator that is responsible for pushing the existing internal firing components at a specific rate that enables the user to repeatedly fire darts from the blaster. Our solution also includes electronic in-house modifications to the internal components of the blaster so that it can be fired via flipping a switch. These modifications consist of circuits that allow the user to fire the blaster by pressing a button on the handle. In addition, the internal components of the blaster also include rechargeable batteries that power the internal circuitry. These rechargeable batteries enable the user to continue using the blaster for longer periods of time without having to stop playing to charge the blaster and eliminate the need to replace batteries. Moreover, these modifications address complications experienced by users with VACTRL including dexterity and grip strength as our solution will enable the user to operate and fire the blaster more easily. The configurations of the components and alterations are shown in the image above. This solution is innovative as it addresses complications experienced specifically by individuals with VACTRL, such as the ability to utilize all fingers simultaneously to grip and hold an object and allows these individuals to operate a NERF blaster. Additionally, our solution is inventive because the blaster can be fired without conventionally pulling the trigger, as the user fires the blaster by pressing a button, and because the internal circuitry and rechargeable batteries are unique to this blaster. Ultimately, this solution will lead to social inclusivity and improved mental and psychological wellbeing as it will enable individuals with VACTRL to participate in NERF-associated social situations.
Andrew Ferrer, David Kraushar, Denny Guo, Lauren Hedges, and Patrick Seifert
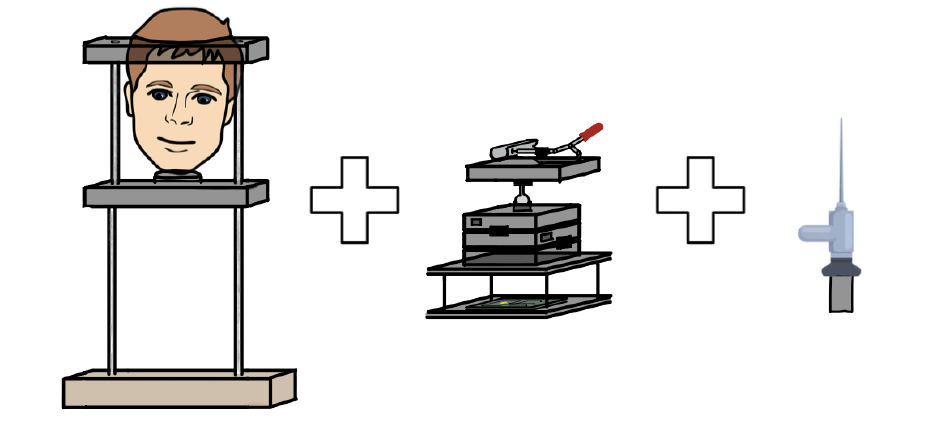
Approximately 20% of Americans over the age of 60 (around 14.8 million people) self-reported sense of smell, olfactory issues. Aging populations often experience neurodegenerative diseases such as Parkinson’s and Alzheimer’s Disease, which can be associated with degradation in their olfactory senses. Current imaging modalities for measuring cerebral blood oxygenation lack dynamic observations, contain unwanted noisy data, and weak signals due to high attenuation that prevent accurate data from being collected from target locations in the brain. The conventional method of delivering NIRS externally causes the photon light to be absorbed by the skin and thick skull, limiting blood absorption. The advantage of internal transnasal NIRS shortens the distance traveled by the photons, improving blood absorption and enhancing data collection. By measuring the scattered near-infrared photons with the detector, blood oxygenation measurements in the brain can be more accurately detected than current external methods. By pairing the NIRS imaging modality with a medical fixation device for the nasal endoscope, the applied NIRS system can be stable in place for extended durations, move within the nasal cavity through fine-tuned adjustments, and maximize the quantity of photons emitted on the target olfactory area. Additionally for safety, the medical fixation device can eject the endoscope through a quick release mechanism to decrease overall patient risk with onset irritation. The innovative aspects of this device include its ability to stabilize a NIRS transnasal endoscope allowing for data collection of cerebral blood oxygenation over a long period of time. The fixation device allows for precise horizontal and vertical adjustments of the endoscope, and includes the ability to change the angle at which the endoscope enters the nasal cavity. The fixture device allows for quick release of the endoscope in order to protect the patient against abrupt changes in position. The solution directly targets a new way to characterize smell disorders among the aging American population, and can also allow for further research opportunities by linking nasal function and neurodegenerative diseases. The completely novel method of determining neuronal-activity in specific regions near the nasal cavity through NIRS transnasal endoscopy could eventually be further optimized for various regions of the brain, outside of the olfactory system.
Molly Dye, Alison Landman, Kunlun Wang, Tianyin Xu, and Xihui Zhao
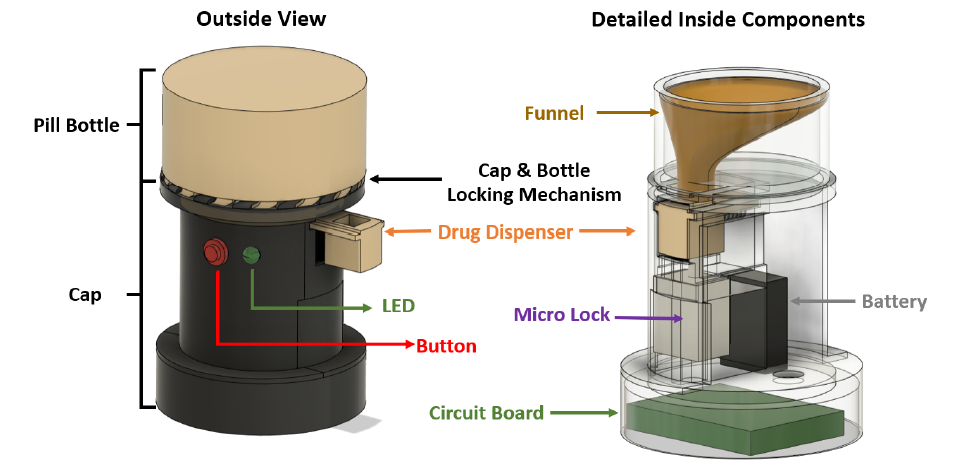
Opioids are pain relief drugs that are often prescribed after surgery or severe injury. If people in the United States who received prescriptions in 2020, 43.3 percent of them were prescribed opioids. In 2021 opioid-related deaths were almost 5 times that in 1999 with nearly 17,000 overdose deaths involving prescription opioids. Existing solutions addressing opioid overuse focus on how to suppress or reduce cravings in those who are already addicted, such as using medication and psychosocial therapy, focusing only on treating opioid use disorder (OUD) once addicted, while there is no efficient method to prevent OUD from developing. Presently, there is no effective way to prevent patients from prescription opioid misuse or overuse, and thus avoid addiction in the first place. We propose a take-home prescription bottle that is programmed to dispense opioid pills at the prescribed rate, which locks the user out of the main pill compartment. This bottle would be filled by the pharmacist and permanently locked once it leaves the pharmacy. By eliminating access to the full bottle of opioids, the patient will be unable to overuse their medication, reducing the risk of dependency. While this device will be unable to cure the addiction of those already experiencing opioid use disorder, the goal of this solution is to reduce the risk of a patient developing an addiction to their prescription opioids. Our solution includes a pill sorting and dispensing system, a programmed timekeeping dispenser with an LED light reminder, and a permanent cap-bottle lock. The device will dispense one pill at a time and be locked for a pre-set time window. Once the countdown from the last dispensed pill ends, the dispenser will unlock itself and be ready for the next pill to be dispensed. New solutions, like ours, provide a brand-new method to directly cut access to the pills, dispensing the correct amount of medicine in the correct time frame, which prevents patients from possible overconsumption or developing a dependency. While there are some devices like Hero and Med-E-Lert implement the use of countdowns to encourage taking medications at the correct times, and in the case of Hero a dispensing mechanism, our novelties include: 1) auto-lock after dispensing the pill to limit patient’s free access towards excessive pills, 2) portable and comparable to the existing pharmacy bottle, and 3) no fixed time window to access prescribed pills once the countdown ends. Implementing these features will help to prevent patients from taking opioids too close to a previous dose or from taking more than prescribed at a given time, which other devices are not currently addressing.
Abbey Painter, Alyssa Hudgins, Alyssa Richards, Arthur Klein, and Neha Rajeev
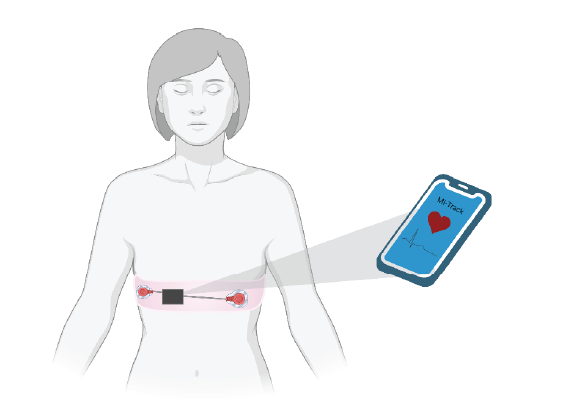
Myocardial Infarction (MI) is the leading cause of death in the US and is responsible for over one million deaths annually. The key to successful treatment lies in early detection and timely intervention. Postmenopausal women are at an increased risk for MI and are less likely to have a timely diagnosis due to unsatisfactory self-identification, among other things. MI presents vastly differently between men and women, where the symptoms in women include fatigue, indigestion, difficulty breathing, and dizziness rather than the classical dramatic pain and alarming cardiac symptoms seen in men. The lack of alarming symptoms and the overlap in symptoms of menopause or other illnesses leads to an increased time before intervention. The increase in MI prevalence in postmenopausal women and their lack of alarming symptoms leaves a gap in early diagnosis and intervention, worsening their outcomes. There is a need for a technology designed to address the lack of detection and elevated incidence of crippling or morbid myocardial infarction in postmenopausal women and other at-risk groups, due to the current failure to quantify the signs and symptoms of a heart attack in these groups. The current market for portable heart monitors includes heart rate monitors and atrial fibrillation detection but lacks information a 12-lead EKG can provide. The device consists of portable EKG leads with a wearable device fit for everyday use. The EKG, integrated into a comfortable chest band, is analyzed with a novel algorithm and alerts the user to any signs of an MI-related event in real time. The user-friendly phone application sends the patient alerts to seek medical attention as needed and serves as a platform to connect patients with a telehealth provider if immediate emergency care is necessary. The device consists of a physical chest band and accompanying software (as seen in the image above). The novelty of this project lies in the application that connects the patient’s EKG data with easily accessible and readable cardiac health information. The novel MI detection algorithm not only alerts to the presence of typical characteristics of an MI such as ST-segment elevation, but also compares the signal to the patient’s established baseline, alerting the user of any significant changes in EKG that could cause need for further examination by a physician.
Iris Layadi, Patricia (Pei-Lun) Chen, Allison Garrard, Shravan Ranganathan, and Dhruv Patel
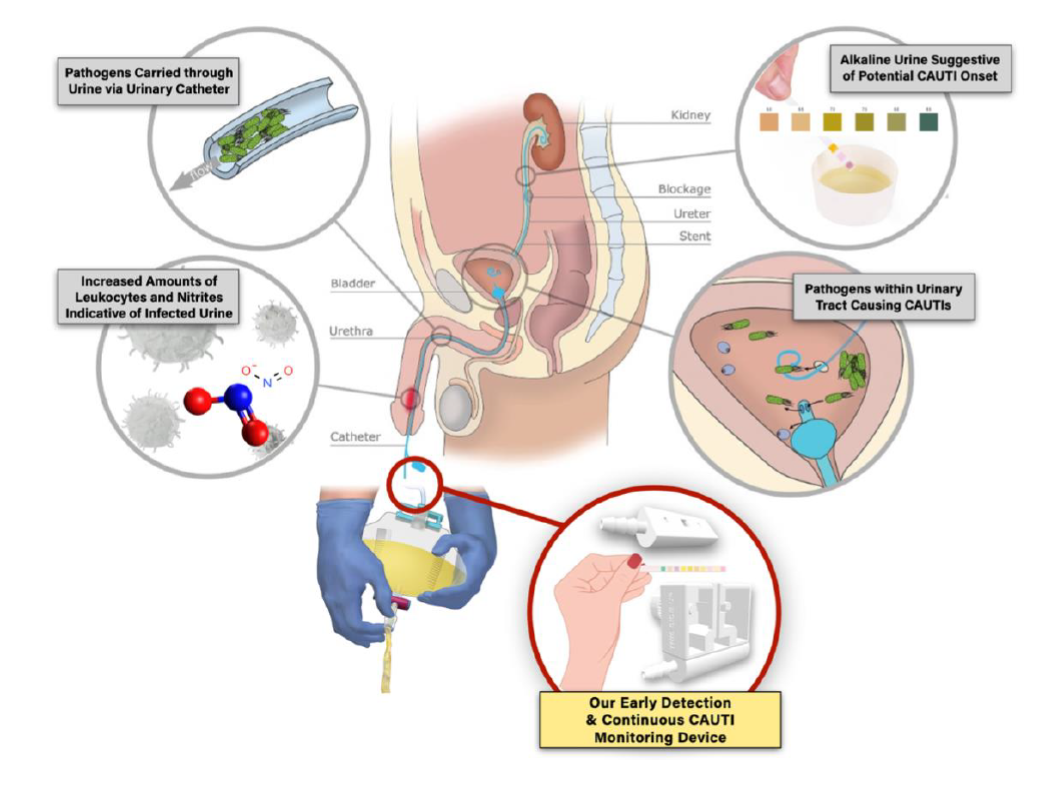
Catheter-associated urinary tract infection (CAUTI) remains the predominant nosocomial infection globally, representing nearly half of all healthcare facility-acquired diseases. Despite advancements in CAUTI management, the frequent use of indwelling urinary catheters, compounded by challenges in maintaining hygiene around the groin area, continues to increase associated morbidity and mortality rates, especially in resource-constrained settings. Currently, CAUTI diagnosis is dependent on clinical symptom monitoring, which often results in diagnostic delays, subsequently causing heightened patient distress, longer hospitalizations, and escalated healthcare costs. Standard testing procedures, though definitive, can take up to three days to produce results. With no current solutions available in the market, we propose a novel UTI test strip dispenser that allows for real-time, autonomous CAUTI detection. Our design is intended to complement laboratory urinalyses and urine culture workups, providing diagnostic support to ease healthcare provider workload and minimize patient costs associated with CAUTI complications. Leveraging the detection capabilities of FDA-approved UTI test strips, our solution brings together 1) an easy-to-use, rotating dispenser that feeds test strips into 2) a module connecting the Foley catheter to the urine drainage bag, and 3) an optical sensor system that delivers alerts to existing staff monitoring portals when color alterations on the reagent pads are detected, suggesting potential CAUTI onset. Separated by a transparent, medical-grade polyvinyl chloride barrier, the reagent pads on the test strips are precisely positioned in front of the optical sensor for accurate leukocyte and nitrite level detection. Clean and waste test strip compartments are incorporated within the device to eliminate risks of leakage and contamination. Additionally, our integrated CAUTI detection and monitoring system can fit seamlessly into nursing workflows and ensure uncompromised subsequent urine tests, encouraging optimized patient care whilst addressing the prevalent challenges of CAUTIs.
































