2018 Senior Design Projects
Early Notification of Heart Attacks with H.A.N.A. - the Heart Attack Notification Agent
The Reduction of Occupational Shoulder Injuries in Sonographers
Modified Inserts for Idiopathic Scoliosis Brace to Reduce Pressure Sores
Quantifying Parkinson’s Symptom Progression with a Toothbrush
Point of Care Cervical Cancer Detection in Low Resource Settings
Automated Syringe Exchange Machine (ASEM) to Improve Access to Syringe Service Programs
Project ESB: Emergency Shoulder Brace
Surgical Pocket Dissector for Cardiac Implants
Wireless Charging for Workstations/Computers on Wheels in a Hospital Environment
Simple and Affordable Imaging Cytometry for Malaria Diagnosis
Autoinjector Initiated Mixing (AIM)
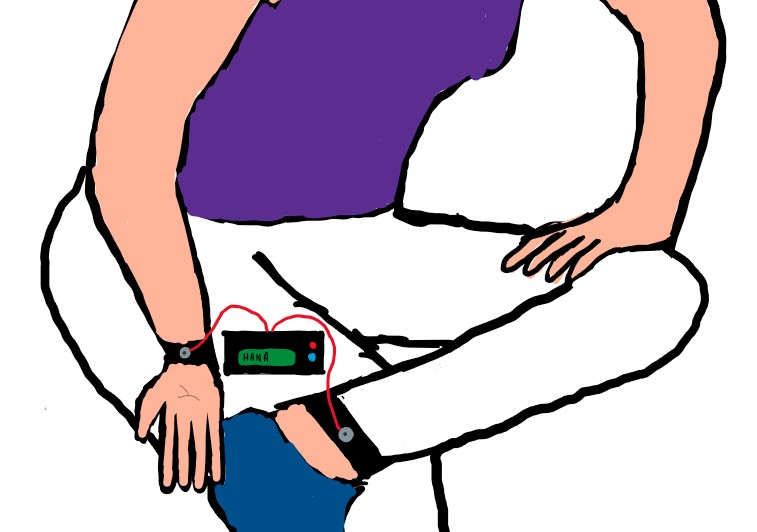
Early Notification of Heart Attacks with H.A.N.A. - the Heart Attack Notification Agent
Anna Fuquay, Madeline McLaughlin, Ahoura Mortazavi, Shazana Nadeem, Carissa Nel
Every year, about 735,000 Americans experience heart attacks and half of those occur outside of the hospital. For those that are experienced outside of hospitals, 88% of patients die. Specifically, when suffering heart attacks, women tend to experience abnormal, few, or mild symptoms such as headaches, muscle fatigue, and nausea. Because these symptoms are atypical and are not normally indicative of a heart attack, these women do not go to the hospital, causing further complications and even death. These complications caused by an undetected heart attack cost the medical industry over $317 billion a year. This issue is amplified because there is not a device available for use outside of the hospital to detect heart attacks in a timely manner without the help or review by a trained medical professional. In order to meet these needs, H.A.N.A, the Heart Attack Notification Agent, is currently being developed. HANA is a portable, at-home device that does not require any technical skills and consists of four subcomponents: (1) the dry electrodes, (2) the electronic components (ECG circuit, algorithm, and arduino), (3) the LCD screen, and (4) the protective casing. Specifically, the user secures one bracelet containing two dry electrodes on her right wrist and one bracelet containing one dry electrode on her left ankle, thus in the lead II configuration to detect the presence of ST-elevation which is indicative of heart attacks. Using an algorithm, the ECG signal collected through the dry electrodes is compared to the user’s healthy baseline and the user is subsequently notified if there is a difference between the signals and thus if the user is suspected to be having a heart attack. Overall, this solution is innovative and different from devices currently on the market because it will autonomously be able to notify users within 5 minutes if their ECG is abnormal and, because of its use of the lead II configuration, it will be able to only detect heart attacks. This will give women ample time to go to the hospital before irreversible heart damage has occurred, ultimately decreasing the number of deaths caused by heart attacks that occur outside of hospitals.

Electrical Stimulation for Trigger Point Detection: A Novel Method to Aid in the Treatment of Plantar Fasciosis
Brett Collar, Hannah Ginsberg, Caitlin Heffner, Dillan Nayee, Caitlin Swanberg
Plantar fasciosis, estimated to affect 2 million people nationally and cost up to $376 million in treatment every year, is a condition in which the plantar fascia tissue that supports the arch of the foot becomes irritated, causing severe pain in the heel. The source of this irritation is typically a localized knot in the nearby muscle, or a muscular trigger point (MTrP). The existing method to identify MTrPs involves a palpation technique to locate the point of pain through verbal and physical feedback from the patient. This practice, based on qualitative data, can be inaccurate and lead to ineffective and unhelpful treatment methods. To improve upon current clinical outcomes, a need exists for an efficient method to accurately identify MTrPs. Properly identifying MTrPs will allow for improved treatment leading to faster recovery times, quicker pain reduction, and less economic burden. Our device has an integrated stimulating/recording electrode that alternates between muscle stimulation and electromyography (EMG) recording in order to obtain an accurate, real-time signal from the muscle. This device is unique in its ability to pass the received signal to an algorithm that differentiates the normal muscle signals from trigger point signals, eliminating the qualitative aspect of current methods. When a trigger point has been identified, a visual and auditory output alert the user. The current and frequency for stimulation can be altered by the user, allowing for deeper penetration, and their values are displayed on a screen. Finally, the device operates on a rechargeable battery allowing consistent use throughout the day. Future human testing and clinical validation will be needed to optimize the accuracy of the algorithm with user interaction. Moving forward, our device can be clinically expanded beyond plantar fasciosis to aid in the treatment of all pains associated with MTrPs, thereby improving patient outcomes.
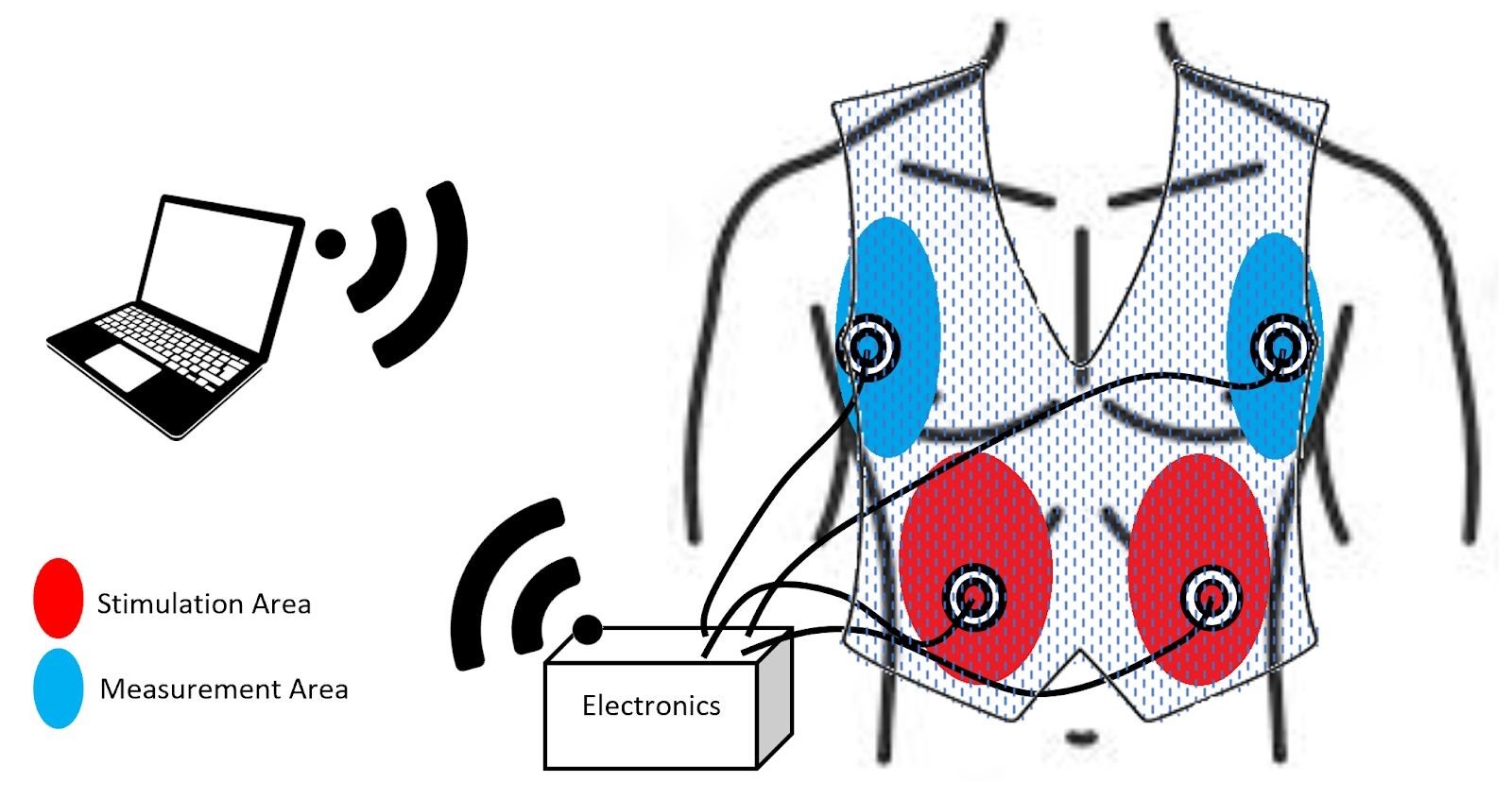
Electroventilator Therapy: Autonomous Closed Loop Control of Diaphragm Stimulation to Induce and Monitor Respiration
Cory Chilcote, Pranith Lomada, Trevor Meyer, Gabriel Ng, Edward Tourney
Human respiration is made possible by the diaphragm: a wide, flat muscle that sits directly beneath the lungs. In certain conditions, there may be a lack of stimulation signals controlling respiration which are normally sent from the brain. This quickly results in a cessation in breathing, leading to a shortage of oxygen and potentially permanent damage to the muscles, kidneys, liver, or even death. Coined “Acute Respiratory Failure” (ARF), this was experienced in 37.5% of total US hospitalizations in 2009, or 720,000 people, and $54 billion in healthcare expenses. Currently, most of these patients are treated with mechanical ventilation, an invasive process which immobilizes the patient and leaves them at risk of infection, along with imposing large expenses and loss of productivity to properly monitor these patients. There is a need for a safer and more time-efficient way to induce breathing in struggling patients and monitor their status in real time. In response, our solution combines two features that had before been mutually exclusive; a closed-loop detection/stimulation response system, and non-invasive methodology. The product is designed to detect lapses in normal breathing pattern and respond with external stimulation of the diaphragm to encourage respiration, ending ARF episodes as soon as they begin. By remaining non-invasive, our product sidesteps infection risk and other related pitfalls brought by mechanical ventilation. Additionally, with the method of a closed loop feedback-controlled system, there is the possibility for more personalized as-needed ventilation to curb patient dependence on a mechanical ventilator as it will only stimulate when help is truly needed. Caretakers will also be able to safely work on other tasks that benefit patients, knowing that individual patients will be safe if they need to leave. The increased productivity means more people can receive better care, improving conditions for all parties involved.
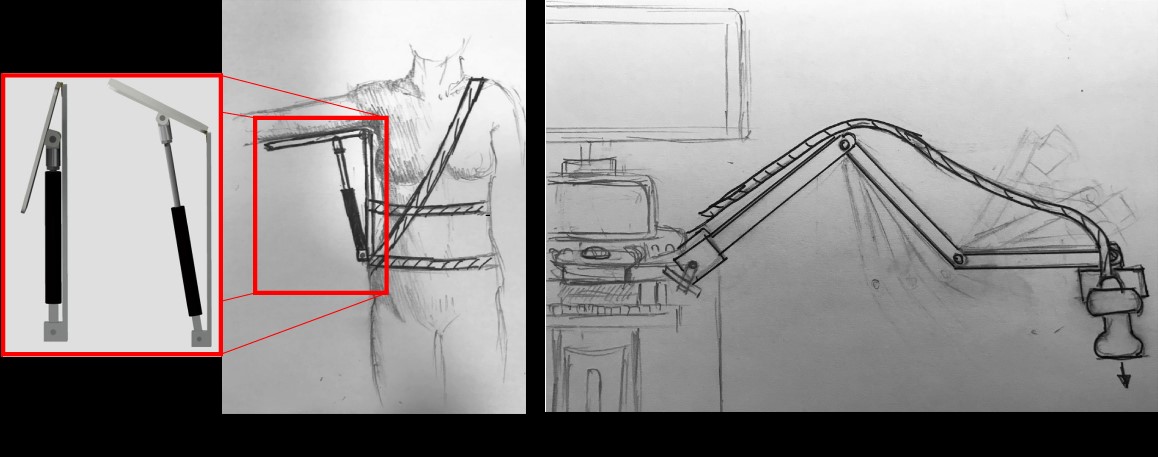
The Reduction of Occupational Shoulder Injuries in Sonographers
Alexander Lai, Grant Tomlin, Christina Lara, Anusha Kumar, Jaibir Khera, Sean Bucherl
In the United States, approximately 90% of sonographers are diagnosed with work-related musculoskeletal disorders (WRMSDs) from performing ultrasound procedures, costing healthcare providers 120 billion dollars annually. These WRMSDs can be localized in numerous areas of the body; however, 76% of injuries are found in the shoulder, making it the most common area for WRMSDs. Shoulder injuries are induced from strain caused by long periods of time in extended arm positions and long periods of time applying forces through a transducer. Therefore, there is a need to prevent shoulder injuries through the reduction of applied forces, and the support of extended arm positions. Our solution seeks to address these needs through the application of two devices that can be used either simultaneously or separately. The first device is an arm support. A gas cylinder raises an arm shelf that the sonographer can rest their arm upon during lengthy procedures. Different than other arm/shoulder support systems on the market, this device can be stowed to the side of the body when not in use and allows support during posterior and anterior arm rotation. The second device is unlike anything on the market and is independent from the first solution. It focuses on decreasing the load on the sonographer’s shoulder by generating the required transducer force through a mechanical system. This solution utilizes an expandable arm that will hold the transducer and apply the force, requiring only positioning by the sonographer. If these innovations succeed in lowering the needed force and supporting the arm and shoulder adequately, both components of the proposed solution can decrease the number of WRMSDs sonographers experience and reduce the monetary load imparted on the healthcare providers.
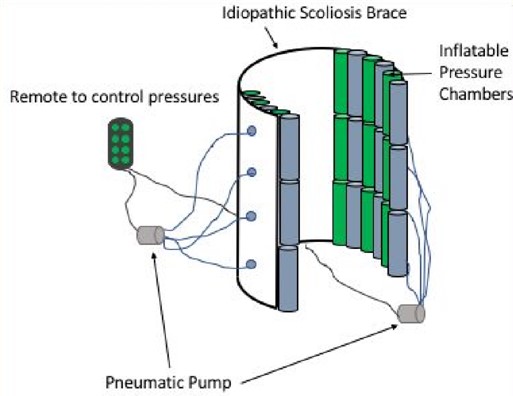
Modified Inserts for Idiopathic Scoliosis Brace to Reduce Pressure Sores
Anna Bird, Alexandra Ford, Caroline Hollier, Nicklaus Iavagnilio, Jacob Thomas
Idiopathic scoliosis is a disease characterized by at least a 10º curvature of the spine and affects an estimated eight million people in the United States. This disease can cause severe complications and damage to internal organs if untreated. Spinal bracing at a young age is the ideal treatment and requires adolescent patients (age 10-15) to wear a rigid brace for up to 23 hours a day as prescribed by a physician. Patients often fail to comply to the bracing regimen due to the risk of skin irritation and pressure sores associated with prolonged brace wearing. In order to increase patient compliance, we will reduce the risk of pressure sores by developing a set of alternating inflatable brace chambers that will be inserted into current brace designs. These chambers will exert corrective forces on the spine, while reducing the occurrence of pressure sores by limiting the amount of time that high pressures are exerted on specific points along the torso. This solution maintains pressures below the accepted standards for pressure sore prevention and is adaptable to the changing curvature of the spine throughout the course of treatment. Our solution’s novel and innovative use of alternating pressure chambers will address the issue of pressure sores. This technology seeks to provide a more comfortable rehabilitation process and will allow patients to be more diligent with their brace-wearing regimen.

Healthier Heart: Non-Invasive, Wearable Blood Pressure Monitor for Improved Treatment of Hypertensive Adults
Antonio Kincaid, Georgia Lawlor, Ryan Prince
Hypertension affects nearly half of the US adult population, characterized by a blood pressure (BP) consistently higher than 130/80 mmHg. Despite uncontrolled hypertension being the most significant contributor to heart disease and stroke, 45% of people do not follow medication or lifestyle regimens because they experience no symptoms. Significant evidence has shown home blood pressure monitoring (HBPM) to be successful in increasing therapy adherence and reducing BP because users are more aware of their condition. Current solutions for HBPM are inaccurate and not portable, creating a need for a wearable device to accurately monitor BP and give medication reminders to control hypertension. The proposed solution, Healthier Heart, is a discreet, watch-style device capable of optically measuring BP on command, along with heart rate and blood oxygen saturation. It is programmed to remind users to track BP and take medication periodically. Optical data is acquired by utilizing a photodiode to sense light reflected off the ulnar artery. When the artery if filled with blood, more light is absorbed resulting in fluctuations, from which the vitals are obtained. A blood pressure cuff on the upper arm is used to occlude the artery, as integrating it within the device fell out of the scope for this project. Vitals are then determined by analyzing patterns in the data to detect the presence of each biometric signal, which is programmed using a microcontroller. This information is then displayed on a small OLED display. Healthier Heart gives its users the option to non-invasively measure BP and other vitals on-the-go and at their discretion. Existing wearable HBPMs cannot be worn continuously and do not give medication reminders, but Healthier Heart uniquely allows these features in conjunction with blood pressure. Successful implementation of Healthier Heart should encourage hypertensive patients to regularly track BP and promote stricter adherence to medication. Lowering blood pressure will reduce the risk of negative outcomes, enhancing quality of life and longevity.
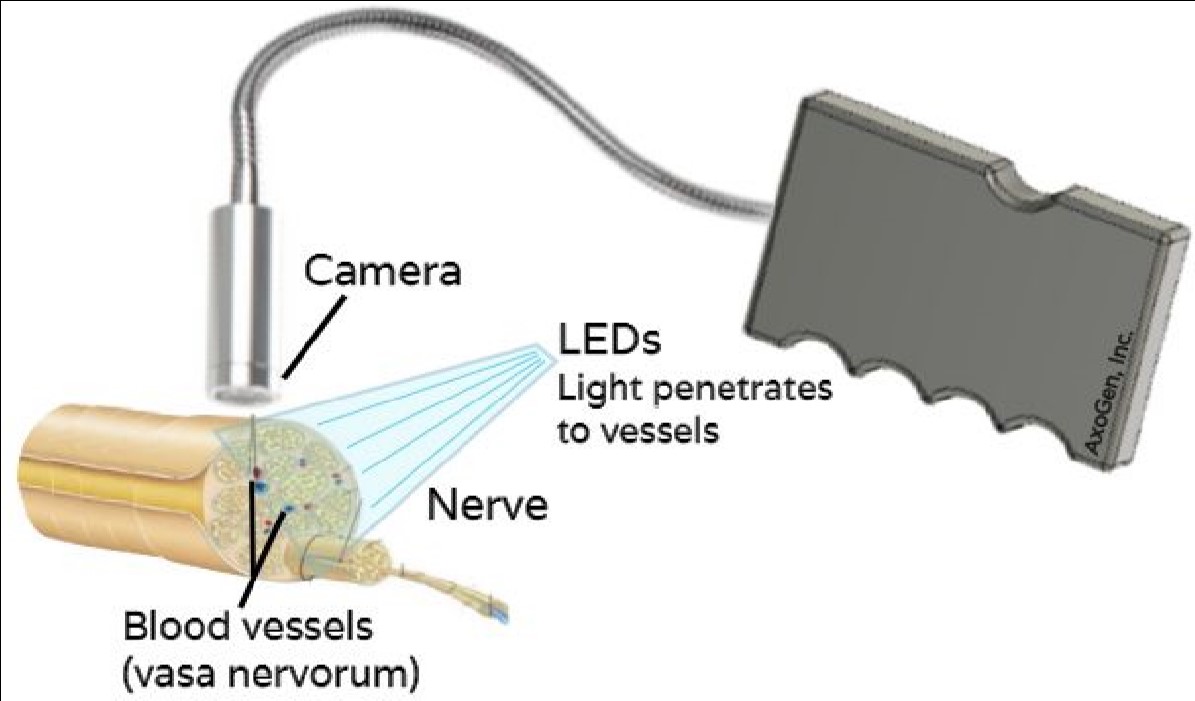
Nerve Viability Detection: Surgical Tool to Image Healthy Blood Vessels in Nerves using Near Infrared Light
Marie Schwartz, Alita Miller, Monica Chanda, Liam Holzer, Christina Hendren
When performing nerve repair, trained surgeons typically evaluate nerve health by eye. Other diagnostic tests, such as nerve conduction velocity and electromyogram tests, require electrical stimulation and take around 30 minutes. Furthermore, the exact location of where the healthy nerve ends and the damaged nerve begins is not always clear. The current standard could cause surgeons to attach a graft on an unhealthy portion of nerve, greatly compromising its performance. Annually roughly 900,000 Americans will require surgical intervention for nerve injury, so a more precise method would have a large impact. Evaluating the blood flow within a nerve will localize its healthy and damaged portions. There is a need for a device which allows surgeons to detect the microvasculature within nerves to evaluate its health and functionality before graft placement. Near-infrared (NIR) imaging is currently being used to visualize blood vessels. This high wavelength light can travel deep into tissue and is absorbed by hemoglobin. Thus, it can be used to image the vasa nervorum, blood vessels that supply nutrients to nerves, as a diagnostic test for nerve tissue viability. These vessels can be as small as 50µm in diameter. Therefore, the ideal solution should detect 50µm blood vessels without requiring a contrast agent or increasing the duration of the surgery. NIR has not been used before to assess nerve viability or to reach such high resolution. Our solution will comprise of a handheld device with light emitting diodes (LEDs) surrounding an NIR detecting camera, allowing for a real-time video stream to an external monitor. With NIR imaging, the surgeon can make the most efficient use of existing healthy nerve to increase graft performance and patient recovery.
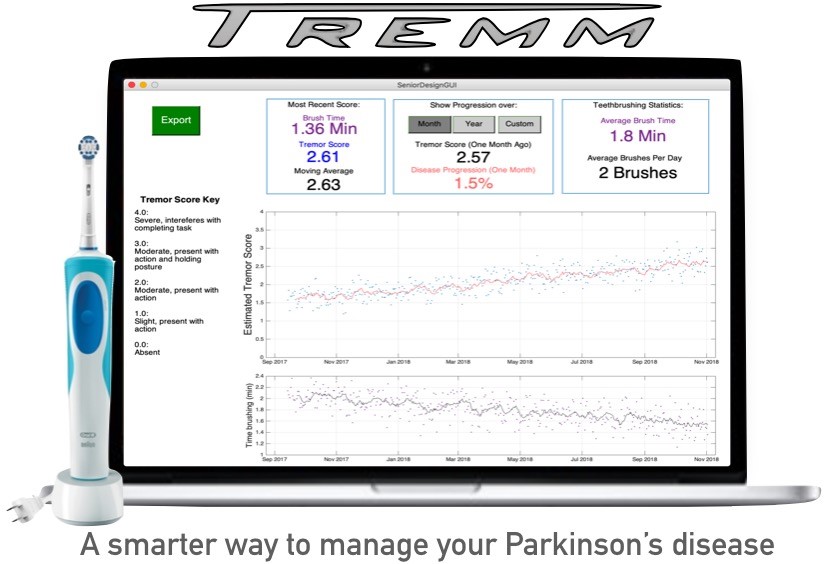
Quantifying Parkinson’s Symptom Progression with a Toothbrush
Carson Rivard, Adam Killeen, Tyler VanDyk, Jon Gaide
Parkinson’s Disease (PD) is a neurological disorder that affects over 10 million people worldwide. Symptoms include motor dysfunction, particularly tremors, and mental impairments that can be treated pharmacologically in early stages. The drug dosage that a patient receives is heavily dependent on the severity of their symptoms, which is difficult to evaluate quantitatively. Often, patients receive an inappropriate prescription because of this difficulty. There exists a need to quantify the severity and progression of Parkinsonian symptoms in order to deliver more appropriate care to PD patients. The solution provided here consists of a motion tracking system embedded into an electric toothbrush. Patients’ hand motion, which can indicate symptom severity, is tracked using an accelerometer and gyroscope while they brush their teeth. The data is processed through spectral analysis to obtain a single metric describing the severity of their symptoms, and these metrics are tracked over time. The result is a data set representing quantified symptom severity every time the toothbrush is used. Similar solutions have been proposed and sold, but all current solutions have difficulty for one of two reasons: Continuous motion data collected from wearables is difficult to interpret, and attempting to collect data during standardized tasks results in low patient adherence. This solution is unique in that it collects data from a standardized task that is already a part of a patient’s daily routine, thus avoiding the two issues discussed above. The data can be viewed by patients so that they better understand the severity and progression of their disease, and can also be used by clinicians to grant deeper insight into the patients’ symptoms, and thus deliver more appropriate care. In doing so, this solution will reduce the occurrence of improper prescriptions for PD patients.
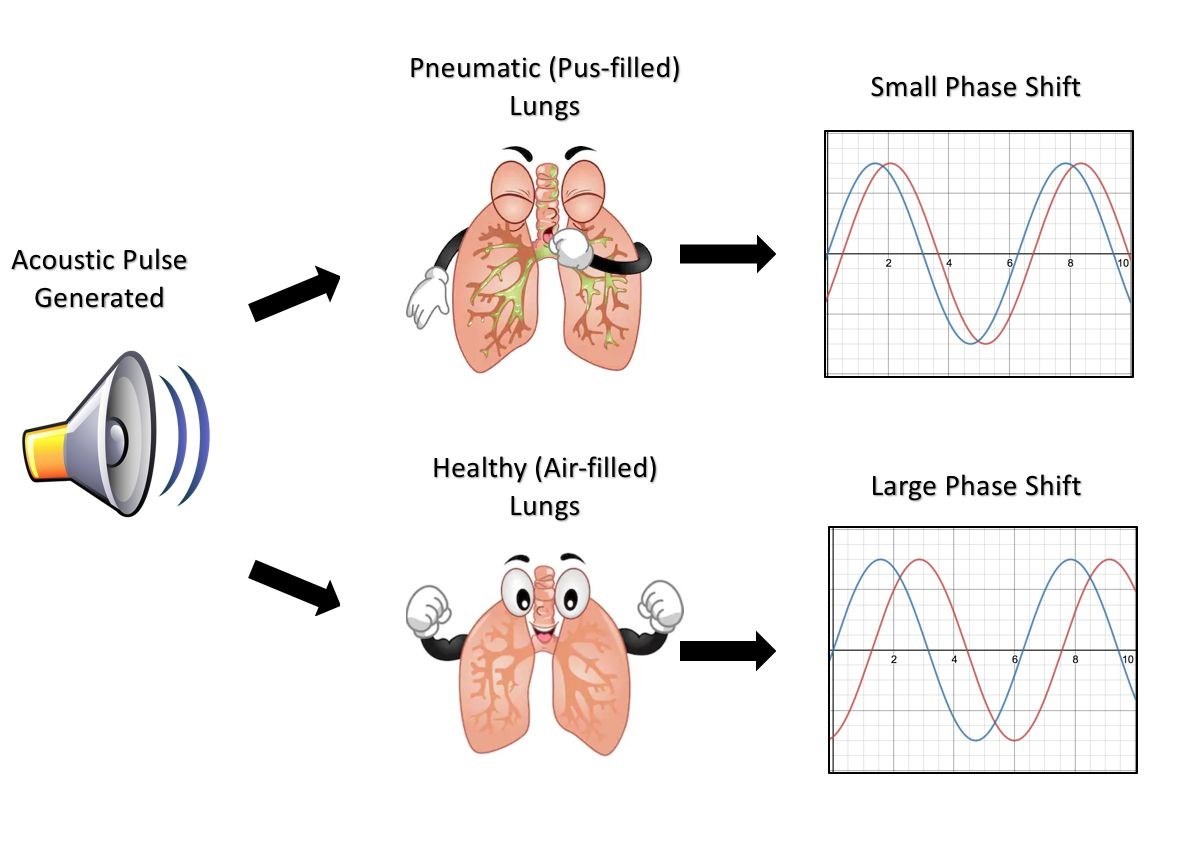
Sound of Pneumonia: Pioneering Acoustic Phase-Shift Detection for Pediatric Pneumonia Diagnosis in Sub-Saharan Africa
Benjamin Brenner, Clayton Culp, Julia Jacob, Logan Rubin
Each year, 650,000 children in Sub-Saharan Africa die from pneumonia, accounting for one in five deaths under the age of 5 in this region. Many of these deaths are caused by a failure to initiate the proper anti-pneumonia treatment protocols, a decision often precipitated by a misdiagnosis of the patient’s pneumonia as malaria or other diseases. Upwards of 45% of pediatric pneumonia cases in Africa are misdiagnosed as malaria due to two compounding factors: the inability of rural clinics to access sophisticated diagnostic technologies and the scarcity of properly trained healthcare professionals. Therefore, we have identified that there is a need for an inexpensive, yet accurate method of diagnosing pneumonia which can be used in rural clinics by minimally trained healthcare workers, including those often deployed by non-governmental organizations (NGOs). Our team has developed such a device that utilizes acoustic signals passed through a patient’s chest cavity to detect the presence of fluid in the lungs; the underlying pathophysiology of pneumonia. The device uses a piezoelectric speaker to generate a 1 kHz tone on the anterior side of the chest which then travels through the patient’s chest and lungs before being subsequently collected on the posterior side by a microphone. A microcontroller is utilized to compare the phase of both the generated and collected audio signals and then determines if the signal has passed through an air-filled or fluid-filled lung. Initial testing has shown a detectable difference in phase between a signal that has passed through a wetted porous sponge representative of a pneumatic lung versus a dry porous sponge simulating a healthy lung. If further developed and deployed, our device stands to greatly decrease the fatality rate of pediatric pneumonia in Sub-Saharan Africa by ensuring pneumonia patients are appropriately diagnosed and administered the proper treatment.
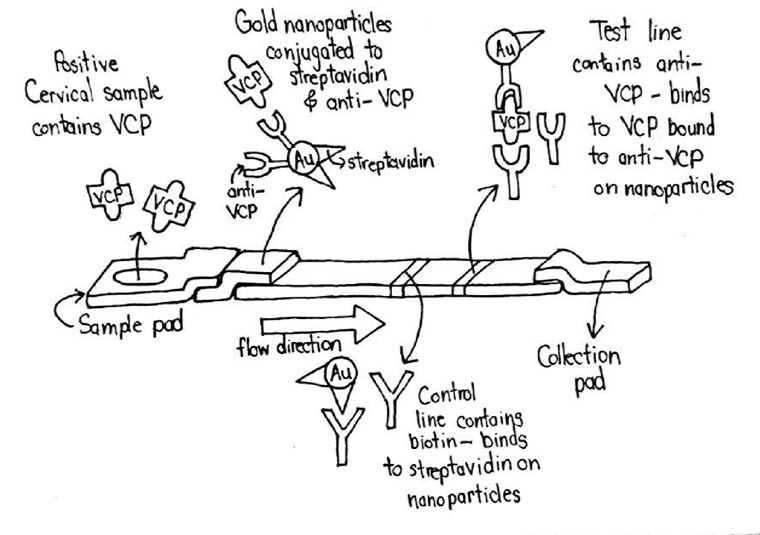
Point of Care Cervical Cancer Detection in Low Resource Settings
Sam Adams, Benjamin Moy, Emilie Newsham, Joseph Sawyer
Cervical cancer is a major problem in the developing world. Women in Zambia are almost four times as likely to develop cervical cancer as women in the US and are more than six times as likely to die from it. This is because the cervical cancer screening techniques used in the developed world are inaccessible to people in low resource settings. Currently used screening techniques for cervical cancer in the developing world involve applying acetic acid or Lugol's iodine to the cervix, which causes cancerous lesions to turn white, and then visually inspecting the cervix. Visual inspection methods give subjective rather than objective results, making them highly dependent on the person administering the test and giving poor sensitivity and specificity. In order to better detect cervical cancer in low resource settings, a lateral flow immunoassay (LFIA) device was developed. LFIAs are commonly used for diagnosis of many diseases in developing countries because of their low cost and ease of use. The LFIA developed by the team is able to detect cervical cancer using valosin containing protein (VCP). VCP was recently discovered by the team’s mentor, Dr. Mohammed, to be present in cervical cancer lesions. As shown in the diagram above, a sample from a cervical swab is added to a paper test strip. As the sample flows through the paper membrane, any VCP present binds to antibodies conjugated to colorful particles. The bound sample then binds to test and control lines further down the test strip, creating colored lines which indicate a positive test result. Detecting VCP in this way will rapidly and inexpensively deliver accurate, objective results. This device will make detecting cervical cancer in low resource settings at the point of care as simple as using a pregnancy test.
K-Plus Measurements: Measuring Serum Potassium Levels at Home for Congestive Heart Failure and End Stage Renal Disease Patients
Jack Jaeger, Elly Lambert, Joey Shin, Shelby Skidmore, Quinn Stillson
Patients suffering from congestive heart failure (CHF) and end stage renal disease (ESRD) are at an increased risk for emergent hospitalization due to unstable potassium homeostasis. According to the National Kidney Foundation, of the 660,000 ESRD patients in America, 24% will require emergent dialysis sometime in their life due to acute hyperkalemia, a condition of high blood potassium levels. Additionally, recent clinical research has shown that 50% of CHF patients taking diuretics will suffer from hypokalemia, a condition of low blood potassium levels. These patients require consistent monitoring and frequent trips to medical laboratories for blood draws as there are no current options to monitor potassium concentration in the blood at home. K-Plus measurements is an at-home device which allows patients to measure their potassium levels on a more frequent basis to better detect trends and eliminate emergency hospitalization. From a sample of blood, measurements will be made by a sensor that detects light intensity emitted from the bond between potassium and a selective fluorescent marker. Moreover, this method includes innovative aspects which allow for an unprecedented, accurate at-home potassium measurement through the elimination of biological artifacts that would disrupt the fluorescence, notably bilirubin in the blood. This device shows promise toward bettering the quality of life for patients with CHF and ESRD by reducing re-hospitalizations, decreasing the likelihood of emergent dialysis, and providing additional data to limit potentially large fluctuations in potassium levels.

Laparoscopic Training Model: A Simulation Training Device to Assist Medical Professionals in Identification of the Cystic Duct
Zain Clapacs, Priya Khimani, Sui Shen, Vyaas Shankar
Laparoscopic cholecystectomy surgery is the removal of the gallbladder which is the most commonly performed abdominal operation in the US with over 750,000 per year. The rate of severe biliary injuries post laparoscopic cholecystectomy was found to be 0.4%-1.4% even after two decades of technology and innovation in this field. Fifty percent of all laparoscopic procedure-related injuries are caused by misidentification of the cystic duct, leading to serious damage to the bile duct. If the user cuts the common bile duct versus the cystic duct, there can be severe patient complications. Currently, there is no high fidelity model for laparoscopic cholecystectomy to focus specifically on preventing bile duct injury. The current training models either focus on a skills-based approach or virtual reality simulation. The skills-based models do not give exposure to various anatomical situations and the virtual reality simulators have a high initial purchasing cost making it not accessible to a wide variety of institutions. Therefore, our team has developed a solution that addresses these gaps by targeting laparoscopic cholecystectomy procedures and focusing on providing different anatomical variation. Additionally, preventing the common misidentification error at a lower cost. In order to create a novel model, our team has identified three major subcomponents: realistic tissues and organs, modular duct anatomy, and cystic duct detection system. The realistic tissues and organs will be made of silicone rubber and mechanically tested to simulate properties of organs in the procedure. The duct anatomy will be provided in six different variations to simulate realistic differences in patient anatomy. The various duct anatomy will allow the user to switch out duct anatomy to practice on rare cases. The duct detection system is the electrical component that will notify the user when the correct duct (cystic duct) has been identified through auditory feedback. In addition to these three major subcomponents, the model will also be representative of the size of the abdomen, be accessible for common-sized laparoscopes, and allow the user to use their non-dominant hand to complete the procedure. This training model will ideally be used in surgical training centers, medical schools, and hospitals to assist surgical training residents as well as experienced physicians in prevention of the misidentification error. This will introduce residents to more challenging situations and practice cystic duct identification to better prepare them for real-world conditions.
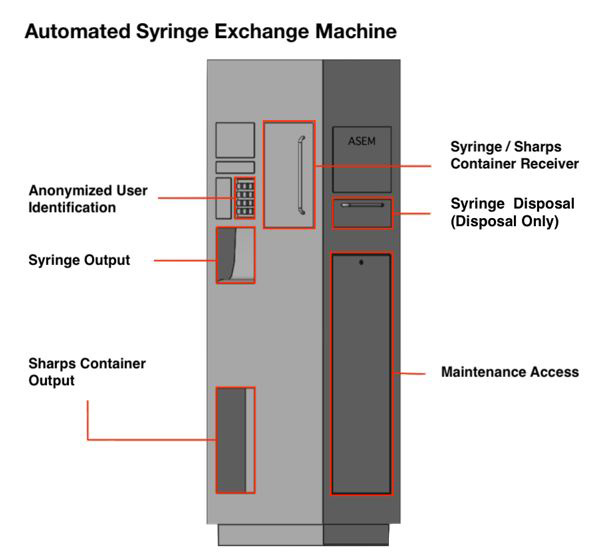
Automated Syringe Exchange Machine (ASEM) to Improve Access to Syringe Service Programs
Barrett Davis, Tavis Logan, Joseph MacDonald, Madi Rogers
Needle sharing among people who inject drugs (PWIDs) is a high-risk behavior directly attributable to blood-borne pathogen exposure and venous injury. In 2015 alone, needle sharing was identified as the root cause behind one of the worst documented outbreaks of HIV among IV users [in the US] in the past two decades, following over 140 documented cases of HIV infections in Scott County, Indiana. Current methods for reducing the incidence of needle sharing -- needle exchanges or syringe service programs (SSPs) chiefly among them -- accomplish this goal by providing free or subsidized access to clean injection materials (syringes, sterile water, alcohol wipes, etc). However, this approach fails to fully cover those who stand to benefit most, with only half of the estimated one million active PWIDs reporting using an SSP in the last year. Though SSPs are incredibly effective at reducing the risk of needle sharing, due to issues with program costs, administrative barriers, and logistical constraints, they remain an inadequate wholesale method for reducing needle sharing. There exists a clinical need to (1) increase the operational hours of SSPs and (2) decrease per-user operational costs. The proposed solution comes as an Automated Syringe Exchange Machine (ASEM), which could be operated remotely, without the need for direct healthcare provider supervision or time, 24/7. A user registered with a local SSP would enter a four-digit pin to access the ASEM, verified with an online user database, before being given the opportunity to deposit their sharps container into the receiving door or disposal door. Should they enter sharps into the receiving door, the ASEM would weigh the deposit and calculate the number of needles deposited in order to output an equal number of deposited needles to establish a desired 1:1 needle exchange ratio, as well as offer a new sharps container, if needed. All input and output data will be tracked in order to analyze maintenance frequency in parallel with the user database to consider frequency of linkage to care. With the need for increased access to materials provided by SSPs as well as the per-user operational costs of these programs, an autonomous, remotely-accessible device that provides syringe exchange services at all hours, in conjunction with recommended visits to a local SSP or to relevant government agencies such as a local health department, will not solve the opioid epidemic but will contribute to the control of the spread of infectious diseases while providing access to rehabilitation and other medical services.
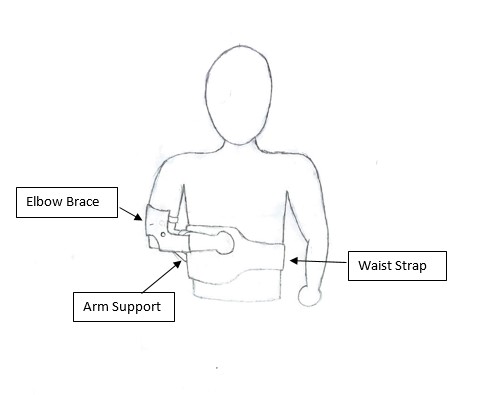
Project ESB: Emergency Shoulder Brace
Filip Szeszko, Timothy Yap
Each year about 71,000 shoulder dislocations arrive in Emergency Departments in the United States alone. Of those, approximately 10,000 are transported by ambulance. Each of those 10,000 patients needs to have their shoulder stabilized for transport as most EMS services are not authorized to reset shoulders on site. However, there is currently no accepted standardized method of stabilization and first responders are forced to create makeshift splints or slings out of available materials in their vehicles. These are often rudimentary, ineffective, and can lead to complications for the patient if they fail in transport. These complications can range from further damage to ligaments and tendons to the formation of blood clots which then travel throughout the body. Therefore our team is developing a shoulder support brace that will provide first responders with a reusable standardized method of shoulder stabilization and immobilization. This device will consist of three components: an elbow brace, a waist strap, and an arm support. Working together these components will take the load off the shoulder and apply it to the torso, while immobilizing the dislocated arm. With an articulated arm support, our brace would be highly customizable to fit each patient’s needs and improve their comfort during transport. In order to accommodate as many patients as possible, the device is being designed to support as much as 20 pounds. Since the arm makes up about 5% of a person’s weight, this would allow us to treat most of the human population. While there are currently marketed devices with a similar design to ours, they are indicated for use after resetting the arm while the limb recuperates. Furthermore, many of these devices are meant for use on one side of the patient’s body, requiring healthcare providers to purchase multiple units of the device to cover their possible needs. Our device is meant to eliminate this concern for first responders through its design for universal application to either side of a patient’s body. In conclusion, our device will reduce variability and error inherent to shoulder dislocations leading to reduced risks to patient health and improvements in capabilities for EMS services.
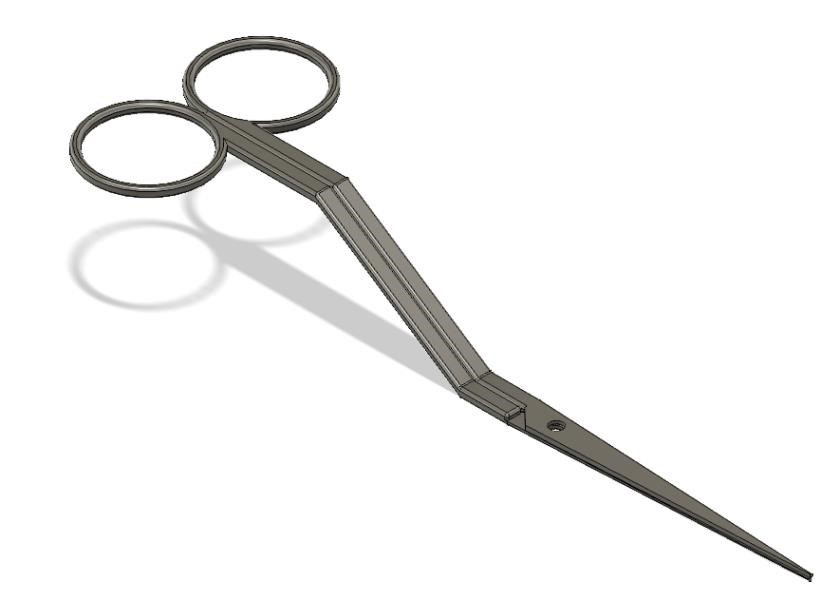
Surgical Pocket Dissector for Cardiac Implants
Nikhil Dave, Mahera Husain, Dimitri Pecoski, Ashlyn Twibell
Over the years, there has been in increase in procedures involving implantable devices. In 2009, according to the World Society of Arrhythmias, 133,262 Implantable Cardioverter Defibrillators (ICDs) were implanted in the U.S., an increase of 12% from 2005. The current gold standard for pocket creation is to hold these implantable devices is a combination of blunt dissection with fingers and electrocauterization. The problem with using blunt dissection is that there is no clear method to create precise pocket sizes. If a pocket is not the proper size, it could either lead to fluid accumulation which results in infection or the implant can erode through the skin leading to potentially preventable life-threatening complications. There is a need for a device that can create surgical pockets for ICDs that are implant-size specific and reduce the incidence of infections caused by bleeding. This device will be used by cardiologists and interventionists in the United States. The proposed solution combines the previous standard of using the blunt dissection technique with a new device that mimics finger dissection and establishes hemostasis using a D-Stat drug coating. This device is handheld and spring-actuated to create precise volumetric size within the tissue (Figure 1.). While surgical implantation devices exists, there are no current solutions for the precise and accurate pocket creation for larger implants. By steering away from the traditional use of electrocautery, our device is innovative by limiting seroma genesis while properly establishing hemostasis during the procedure. The implementation of this device has the potential to have a significant impact on cardiac implant procedures by standardizing the pocket created, thus reducing time and complication rate. In the future, this surgical pocket dissector is foreseen to be a standard tool in a physician’s arsenal.

Wireless Charging for Workstations/Computers on Wheels in a Hospital Environment
Mukunda Aithal, Hayley Salaman, Adam Scott, Matthew Tharp
Hospitals and long term care facilities utilize mobile workstations nicknamed workstations on wheels (WOWs) or computers on wheels (COWs) to manage electronic medical records. Some issues that health care professionals have reported with these devices include short battery life, overall device bulkiness, failure to keep devices charged, and/or difficulty finding available electrical outlets or already charged workstations. Nurses, the primary users of these devices, have experienced the highest incidence of lower back issues within the medical profession, and the strenuous stop-and-go charging process of charging these workstations is a potential major contributor to the physical strain induced to nurses while utilizing these devices. To address these problems, we have considered an overhaul to the mechanism in which COWs/WOWs receive power in a hospital environment. We propose the development of wall-mounted wireless charging “pads” placed throughout the hospital to transmit power to a wireless charging adapter. This approach to overhauling the charging mechanism for these devices requires strategically placing power transmitting pads throughout the hospital along with their counterpart receiving pads for each workstation. Our system design includes a unique amplifier circuit combined with transmitting coils which are located on the transmitting pads. There is also a receiver coil leading to the receiver circuit located on the workstation which will charge the battery. To charge efficiently at a distance, we have pursued Rezence-certified magnetic resonance coupling techniques. This important and innovative solution includes the wireless technology necessary to transmit a power capacity of at least 12.4 Watts at a distance of 0.5 meters to maintain the charge of most computers. Additional aspects of this solution include form factors for the charging pads on the wall and tutorials to facilitate installation. With this approach, we hope to increase workplace efficiency in the hospital by ensuring that most WOWs are charged and usable at all times, thereby cutting down wasted time searching for a charged WOW. In addition, the system may also decrease hospital liability and reduce nurse back injuries by removing the need for nurses to constantly bend over to manually charge these devices.

Simple and Affordable Imaging Cytometry for Malaria Diagnosis
Junjie Chen, Eugene Kim, Yi Wang
In 2016, there were 216 million clinical episodes and over 400,000 deaths caused by malaria worldwide, threatening almost half of the world population. Most cases happen in resource- limited regions such as Africa and South Asia. Thus, diagnosis of malaria in these regions poses a tremendous challenge since current diagnostic methods are either too expensive or not sensitive enough. Additionally, asymptomatic malaria infections that are left undiagnosed serve as “reservoir” of potential outbreaks, making it nearly impossible to eliminate malaria infections in a region. There is a need for a diagnostic device that is sensitive enough to detect asymptomatic infections while being cheap enough to be used in resource-limited settings. Flow cytometry has been proven to be an effective and highly sensitive method in malaria diagnosis. Our team adopts an adapted form of flow cytometry, known as imaging cytometry, to identify the morphology of red blood cells in a slide. This will allow the device to find malaria parasites using fluorescence imaging and cell morphology to pinpoint parasitic nucleic acid within red blood cells. This simple solution will be made widely available to laboratory and institutions in regions most deeply troubled by malaria infection. The imaging system, with its high sensitivity and specificity, will enable detection of asymptomatic malaria parasites inside red blood cells with minimum resource requirements. With the diagnostic power of our device, new malaria infection cases will be greatly reduced and even completely eliminated not far in the future.
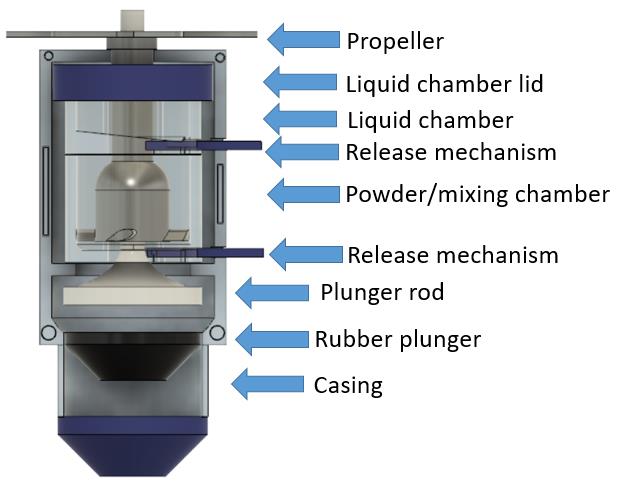
Autoinjector Initiated Mixing (AIM)
Jessica Bendell, Dana Moryl, Xiaohong Tan
Autoimmune disease medications that require reconstitution before injection can be overly complex, leading to users forgetting steps or improperly mixing their medications. Unfortunately, there is not an autoinjector device that contains the all-in-one package: mixing, actuation, injection, and needle retraction. Most autoimmune disease treatments contain liquid drug product and are prescribed in autoinjectors due to their ease of use, as compared to at-home reconstitution or pre-filled syringes. However, storing drug product as a liquid lead to stability problems such as decreased drug activity (drug potency and efficacy) and greater byproduct generation. Thus, there is a need to store the drug powder and diluent in separate chambers until time of use and perform reconstitution within the autoinjector to provide increased stability of drug product and improve overall patient experience. This autoinjector pen contains separate compartments for the liquid and the drug powder. The device then allows for patient initiated reconstitution, and deposits the medication above the syringe for injection. A separate mechanism will extend the needle and intramuscularly inject the medication into the patient, which will be developed by a separate team at Rose Hulman. After the injection is completed, the needle will be retracted into the pen. AIM has the capability to mix and deliver the DP and retract the needle in an autoinjector without excessive steps of reconstitution. Currently, there are no autoinjectors that address the need to keep the drug powder and diluent separate until administration. AIM can greatly improve the lives of 23.5 million autoimmune patients in the US who need regular administration of medication. By eliminating long-term liquid DP storage and reducing reconstitution steps, this device can improve their quality of life.
