2019 Senior Design Projects
NUTRIX - Breast Milk Analyzer to Improve Infant’s Individualized Fortification
Monitoring Clearance of Urea Across a Dialyzer during Continuous Renal Replacement Therapy
Nerves of Steel - A Device to Atraumatically Handle Nerves
Peripheral Monitoring Using a Multimodal Biosensor for Rapid Detection of Congestive Heart Failure
Blood Loss Quantification Method for Rapid Diagnosis of Postpartum Hemorrhage (PPH)
Wireless Powering of Implantable Devices using Current Clinical Frequency Bands
WOODS - Wearable Opioid Overdose Detection System
Low-Cost Motorized Knee Prosthesis for Above Knee Amputees
Sterile Field Break Detection Using a Capacitive Sensor System
The Bee’s Knee: An Autonomous Knee Brace for ACL Injuries
At-home Self-collection of Cells for Cervical Cancer Diagnosis
OnePill: Redesigning the Prescription Bottle for Pediatric Medication Safety
Colonoscopic Tool Organization and Retraction Automation
Automatic Detection of Obstructive Sleep Apnea Using a Single Lead ECG Signal
NIRVVE - Nerve Viability Detection: A Method to Image Blood Vessel Health in Nerves Using Near-Infrared Light
Joe Ancel, Connor Koelsch, Cole Wysocki, Ryan Lindsey
 Peripheral nerve surgery involves removing damaged nervous tissue and introduce a graft which allows the nerve to regrow properly. Current methods for identifying damaged nervous tissue rely on a “cut and check” method. Surgeons start cutting around the known, damaged area of the nerve and keep cutting until the nerve starts bleeding a normal amount. This normal blood flow indicates that the nerve vasculature is undamaged, functional, and healthy. This current method is not accurate and results in the removal of healthy nervous tissue which can result in post-surgery pain, longer recovery time, and even chronic post-surgery complications for the patient. With nearly a million Americans requiring surgical intervention for peripheral nerve injury each year, there is a need for a device that can allow surgeons to non-invasively visualize nerve health during surgery. Current methods of imaging blood vessels within nerves require contrast agents and/or bulky equipment that is not easy to manipulate around such small nerves, especially in a surgical setting. Near-infrared (NIR) imaging is currently used to visualize large blood vessels directly under the skin in devices such as the Vein Finder. The wavelengths of Near Infrared (NIR; 750-2500 nm) can travel deep into tissue. Hemoglobin in the blood absorbs much of NIR light while the surrounding tissue does not. At around 850 nm, the difference between oxygenated hemoglobin and deoxygenated hemoglobin absorbances is maximized. Exposing a nerve to this wavelength of light creates a high contrast image of the nervous tissue and its vasculature. Our solution is a device that utilizes a NIR camera and an LED ring light that floods the imaging area with 850 nm light. The camera and ring light are located at the end of a wand that is easy to hold and maneuver around small objects. The camera is connected to a microcontroller that supports real-time streaming to an external monitor. Both the camera and the ring light are powered by a rechargeable lithium ion battery. Blood vessels within nerves have diameters of 50 microns or larger. For this reason, the camera must be able to resolve objects smaller than this. The combination of these parts allows for a wireless and lightweight device capable of viewing blood vessels, with diameters on the scale of microns, without the use of a contrast agent. With this device, surgeons will maximize efficiency of nervous tissue removal, thus minimizing the patient’s post-surgery recovery time and pain.
Peripheral nerve surgery involves removing damaged nervous tissue and introduce a graft which allows the nerve to regrow properly. Current methods for identifying damaged nervous tissue rely on a “cut and check” method. Surgeons start cutting around the known, damaged area of the nerve and keep cutting until the nerve starts bleeding a normal amount. This normal blood flow indicates that the nerve vasculature is undamaged, functional, and healthy. This current method is not accurate and results in the removal of healthy nervous tissue which can result in post-surgery pain, longer recovery time, and even chronic post-surgery complications for the patient. With nearly a million Americans requiring surgical intervention for peripheral nerve injury each year, there is a need for a device that can allow surgeons to non-invasively visualize nerve health during surgery. Current methods of imaging blood vessels within nerves require contrast agents and/or bulky equipment that is not easy to manipulate around such small nerves, especially in a surgical setting. Near-infrared (NIR) imaging is currently used to visualize large blood vessels directly under the skin in devices such as the Vein Finder. The wavelengths of Near Infrared (NIR; 750-2500 nm) can travel deep into tissue. Hemoglobin in the blood absorbs much of NIR light while the surrounding tissue does not. At around 850 nm, the difference between oxygenated hemoglobin and deoxygenated hemoglobin absorbances is maximized. Exposing a nerve to this wavelength of light creates a high contrast image of the nervous tissue and its vasculature. Our solution is a device that utilizes a NIR camera and an LED ring light that floods the imaging area with 850 nm light. The camera and ring light are located at the end of a wand that is easy to hold and maneuver around small objects. The camera is connected to a microcontroller that supports real-time streaming to an external monitor. Both the camera and the ring light are powered by a rechargeable lithium ion battery. Blood vessels within nerves have diameters of 50 microns or larger. For this reason, the camera must be able to resolve objects smaller than this. The combination of these parts allows for a wireless and lightweight device capable of viewing blood vessels, with diameters on the scale of microns, without the use of a contrast agent. With this device, surgeons will maximize efficiency of nervous tissue removal, thus minimizing the patient’s post-surgery recovery time and pain.
NUTRIX - Breast Milk Analyzer to Improve Infant’s Individualized Fortification
 Infants born prematurely or with certain health conditions may require additional calories to ensure healthy development. The CDC estimates that one in every ten babies was born prematurely in 2017. The milk being consumed by these infants must be fortified to ensure healthy development, but it is not currently individualized per the infant’s needs and the mother’s breast milk content. Instead, NICU nurses currently fortify milk nonspecifically based only on the baby’s birth weight and date. A significant portion of the calories in each ounce of breast milk are contributed from fat, thus it would be important to know the calories from fat in the breast milk to be able to provide individualized and targeted fortification to ensure the infant is receiving sufficient calories. There is no low-cost, accurate, and mobile way to analyze the calorie content of breast milk for breastfeeding mothers and healthcare professionals that provides immediate feedback. Their babies are not receiving individualized milk fortification dependent on their own breast milk content and their babies’ health conditions. Existing solutions are not utilized clinically because they are not sufficiently affordable, accurate, nor portable. To improve individualized fortification and ensure infants are receiving calories based on their condition and the mother’s milk, a breast milk analyzer has been designed to measure and output the calories from fat in a milk sample. There is a need for an effective way to measure the calorie content of breastfeeding mothers’ milk. Nutrix is lower cost, easy-to-use, and clearly and accurately indicates whether the milk serves as sufficient nutrition for their baby and informs decisions about individualized breast milk fortification. The analyzer, named ‘Nutrix’, will be used by doctors, nurses, mothers, lactation consultants, and other professionals to provide targeted fortification. The Nutrix will be an affordable, accurate, and portable solution. The device will consist of a reusable tube to contain the milk sample, a screen displaying kilocalories per ounce from fat secured in an outer shell, which also houses the printed circuit board, the Arduino Uno, and the batteries. These components are all inexpensive and hand-held, thus they will allow for portability and affordability. It will function by sending an electrical current through the milk sample, measuring the sample’s impedance, correlating the impedance values to grams of fat, translating the grams into calories from fat through the use of a standard curve, and outputting the reading to the user on the screen. Overall, Nutrix will provide an affordable and portable option for providing targeted breast milk fortification to improve premature infant outcomes without sacrificing accuracy.
Infants born prematurely or with certain health conditions may require additional calories to ensure healthy development. The CDC estimates that one in every ten babies was born prematurely in 2017. The milk being consumed by these infants must be fortified to ensure healthy development, but it is not currently individualized per the infant’s needs and the mother’s breast milk content. Instead, NICU nurses currently fortify milk nonspecifically based only on the baby’s birth weight and date. A significant portion of the calories in each ounce of breast milk are contributed from fat, thus it would be important to know the calories from fat in the breast milk to be able to provide individualized and targeted fortification to ensure the infant is receiving sufficient calories. There is no low-cost, accurate, and mobile way to analyze the calorie content of breast milk for breastfeeding mothers and healthcare professionals that provides immediate feedback. Their babies are not receiving individualized milk fortification dependent on their own breast milk content and their babies’ health conditions. Existing solutions are not utilized clinically because they are not sufficiently affordable, accurate, nor portable. To improve individualized fortification and ensure infants are receiving calories based on their condition and the mother’s milk, a breast milk analyzer has been designed to measure and output the calories from fat in a milk sample. There is a need for an effective way to measure the calorie content of breastfeeding mothers’ milk. Nutrix is lower cost, easy-to-use, and clearly and accurately indicates whether the milk serves as sufficient nutrition for their baby and informs decisions about individualized breast milk fortification. The analyzer, named ‘Nutrix’, will be used by doctors, nurses, mothers, lactation consultants, and other professionals to provide targeted fortification. The Nutrix will be an affordable, accurate, and portable solution. The device will consist of a reusable tube to contain the milk sample, a screen displaying kilocalories per ounce from fat secured in an outer shell, which also houses the printed circuit board, the Arduino Uno, and the batteries. These components are all inexpensive and hand-held, thus they will allow for portability and affordability. It will function by sending an electrical current through the milk sample, measuring the sample’s impedance, correlating the impedance values to grams of fat, translating the grams into calories from fat through the use of a standard curve, and outputting the reading to the user on the screen. Overall, Nutrix will provide an affordable and portable option for providing targeted breast milk fortification to improve premature infant outcomes without sacrificing accuracy.Monitoring Clearance of Urea Across a Dialyzer during Continuous Renal Replacement Therapy
Christina Bagnati, Kristin Barringhaus, Marisa Hughes, Sara Smith
The current method of measuring dialyzer lifespan by monitoring changes in transmembrane pressure may be inaccurate for continuous renal replacement therapy (CRRT) because it does not consider the effects of protein polarization. Protein polarization reduces the effectiveness of the dialyzer membrane by occluding pores and preventing the removal of waste products from blood. This is detrimental for critically ill patients with acute kidney injury (AKI) who depend on effective CRRT treatment, lasting at least 48 hours, for their recovery. There is a clinical need for a device to measure clearance, or removal, of waste products across a dialyzer membrane during CRRT treatment for AKI patients. The solution must continuously monitor the clearance of waste products by measuring sodium removal, an ion of comparable size to urea, and must be implementable for the duration of the treatment. Impedance will be measured to correlate with changes of concentration. When the clearance of urea, estimated by the impedance of sodium in fluid, becomes too low, the clinician will acknowledge that the dialyzer needs to be replaced to restore treatment effectiveness. This device will impact 200,000 patients in the US annually and will increase the assurance of CRRT effectiveness for critically ill patients.
Nerves of Steel - A Device to Atraumatically Handle Nerves
 Atraumatically handling nerves is a difficult task. Currently within the medical field, surgeons are using forceps to handle nerves during surgery. Unskilled or rushed surgeons can accidentally apply too much force to a nerve causing damage to the epineurium or internal vessels and fascicles. Damage to nerves during surgery can lead to neuropathy that causes weakness, numbness, or pain in the distal regions of the body. In some cases, burning or tingling sensations can arise leaving the patient with discomfort. Studies have indicated that at least 14% of peripheral nerve repair surgeries have poor outcomes where the patient does not regain tactile sensibility or the ability to move against gravity or resistance. In these cases, procedures often need to be performed again, which can be expensive for both the patient and hospital, given that the average cost of a peripheral nerve repair surgery is about $47,000. There is a clinical need for a device to be used in peripheral nerve repair surgeries that gently and accurately maneuvers nerves during procedures while assisting with common nerve entubulations, such as into caps or connectors. Our solution to this problem is a device composed of three components: a vacuum, a handheld portion, and interchangeable tips. The vacuum is a regulated vacuum commonly found throughout hospitals and attaches to the handheld portion providing control over the vacuum force applied to the nerve. There is a sleeve located on the handheld portion that allows for the release of the vacuum when slid up or down. On the distal end of the handheld portion is the tip. The tip contains a small exterior channel on the bottom that allows the nerve to interface easily with the device. Small holes within this channel allow the vacuum to suction the nerve onto the device creating a seal so the nerve can then be picked up and maneuvered during surgery. The tips are interchangeable to allow for different sized nerves to have different size tip channels allowing for easy use. By using this device, surgeons will be able to atraumatically handle nerves, prevent patients from having unnecessary symptoms caused by nerve damage, and save patients and hospitals money by preventing secondary surgery.
Atraumatically handling nerves is a difficult task. Currently within the medical field, surgeons are using forceps to handle nerves during surgery. Unskilled or rushed surgeons can accidentally apply too much force to a nerve causing damage to the epineurium or internal vessels and fascicles. Damage to nerves during surgery can lead to neuropathy that causes weakness, numbness, or pain in the distal regions of the body. In some cases, burning or tingling sensations can arise leaving the patient with discomfort. Studies have indicated that at least 14% of peripheral nerve repair surgeries have poor outcomes where the patient does not regain tactile sensibility or the ability to move against gravity or resistance. In these cases, procedures often need to be performed again, which can be expensive for both the patient and hospital, given that the average cost of a peripheral nerve repair surgery is about $47,000. There is a clinical need for a device to be used in peripheral nerve repair surgeries that gently and accurately maneuvers nerves during procedures while assisting with common nerve entubulations, such as into caps or connectors. Our solution to this problem is a device composed of three components: a vacuum, a handheld portion, and interchangeable tips. The vacuum is a regulated vacuum commonly found throughout hospitals and attaches to the handheld portion providing control over the vacuum force applied to the nerve. There is a sleeve located on the handheld portion that allows for the release of the vacuum when slid up or down. On the distal end of the handheld portion is the tip. The tip contains a small exterior channel on the bottom that allows the nerve to interface easily with the device. Small holes within this channel allow the vacuum to suction the nerve onto the device creating a seal so the nerve can then be picked up and maneuvered during surgery. The tips are interchangeable to allow for different sized nerves to have different size tip channels allowing for easy use. By using this device, surgeons will be able to atraumatically handle nerves, prevent patients from having unnecessary symptoms caused by nerve damage, and save patients and hospitals money by preventing secondary surgery.Peripheral Monitoring Using a Multimodal Biosensor for Rapid Detection of Congestive Heart Failure
Nicolet Elsbury, Maciej Filar, Alex Miller, and Chris Rust
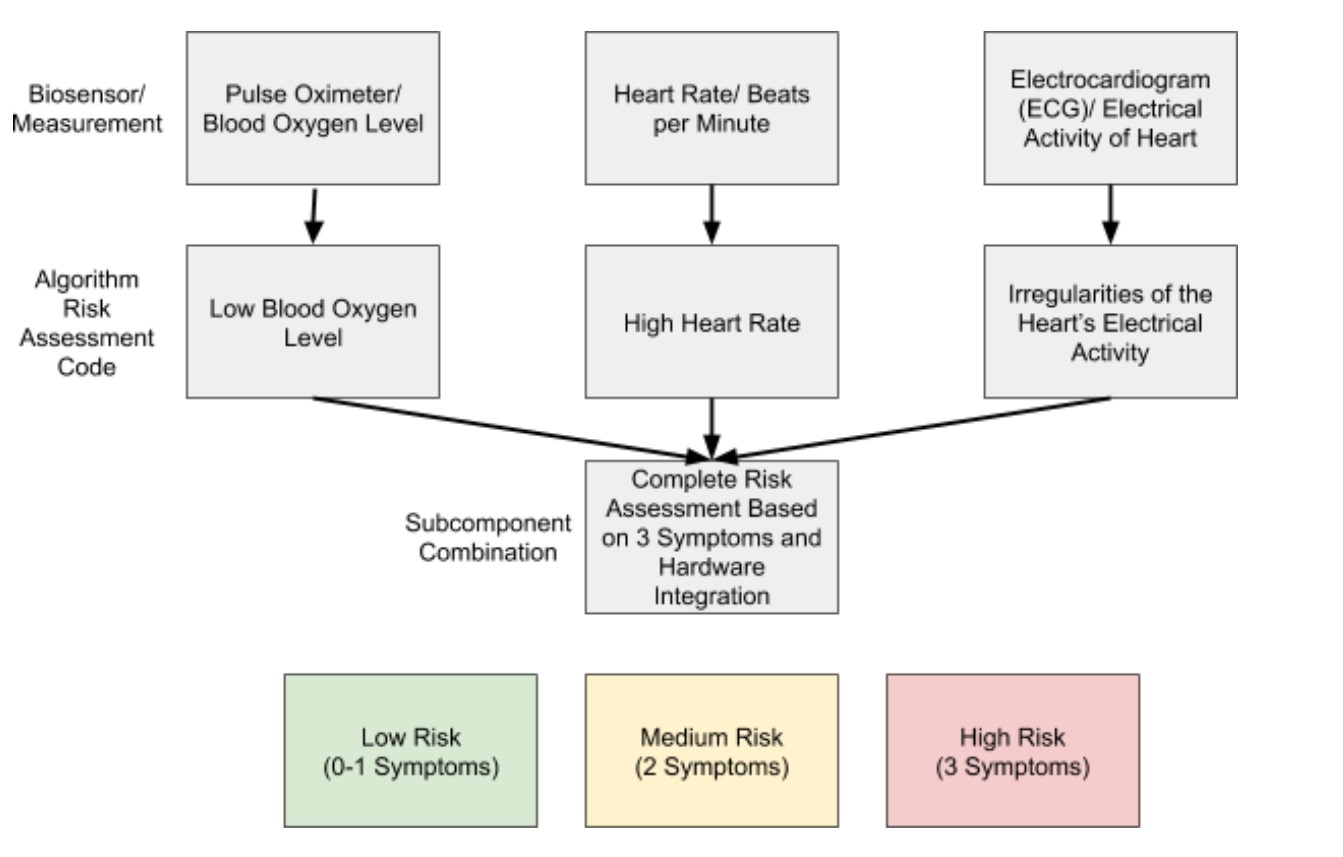 Congestive heart failure (CHF) is a medical condition in which heart muscle is unable to effectively pump blood throughout the body, leading to lower cardiac output and blood oxygenation levels. As one of the most common cardiovascular diseases, CHF affects 5.7 million U.S. adults and contributes to 290,000 deaths per year. Common symptoms of CHF include fluid buildup in the peripheral system, an irregular heartbeat, shortness of breath, and edema, which can worsen as the condition progresses. Currently, diagnosis for CHF is time-consuming and costly as it requires multiple doctor visits, consultation of a cardiologist and potentially additional testing such as magnetic resonance imaging or an invasive myocardial biopsy. There is a need for a rapid, accurate and inexpensive biosensor for an at-home risk assessment of the condition. The device will help reduce cost and patient burden and will decrease the time required for patients to receive treatment. Emerging biosensors that attempt to address these needs fail to use an integrative approach to assess CHF, and when an integrative approach is used, the biosensors are oftentimes invasive, expensive or inconvenient for patients. The new device will be non-invasive and will integrate (1) blood oxygenation level, (2) heart rate and (3) ECG irregularities to provide an overall risk assessment of the disease. A patient will place their palms on the comfortable device platform to retrieve biometric data that will be analyzed by a risk algorithm and output a data summary along with recommended steps the patient should take to better or maintain their cardiac health. Using this approach will help increase accuracy with CHF screening, and will decrease the 855 million dollars spent on CHF yearly.
Congestive heart failure (CHF) is a medical condition in which heart muscle is unable to effectively pump blood throughout the body, leading to lower cardiac output and blood oxygenation levels. As one of the most common cardiovascular diseases, CHF affects 5.7 million U.S. adults and contributes to 290,000 deaths per year. Common symptoms of CHF include fluid buildup in the peripheral system, an irregular heartbeat, shortness of breath, and edema, which can worsen as the condition progresses. Currently, diagnosis for CHF is time-consuming and costly as it requires multiple doctor visits, consultation of a cardiologist and potentially additional testing such as magnetic resonance imaging or an invasive myocardial biopsy. There is a need for a rapid, accurate and inexpensive biosensor for an at-home risk assessment of the condition. The device will help reduce cost and patient burden and will decrease the time required for patients to receive treatment. Emerging biosensors that attempt to address these needs fail to use an integrative approach to assess CHF, and when an integrative approach is used, the biosensors are oftentimes invasive, expensive or inconvenient for patients. The new device will be non-invasive and will integrate (1) blood oxygenation level, (2) heart rate and (3) ECG irregularities to provide an overall risk assessment of the disease. A patient will place their palms on the comfortable device platform to retrieve biometric data that will be analyzed by a risk algorithm and output a data summary along with recommended steps the patient should take to better or maintain their cardiac health. Using this approach will help increase accuracy with CHF screening, and will decrease the 855 million dollars spent on CHF yearly.
Blood Loss Quantification Method for Rapid Diagnosis of Postpartum Hemorrhage (PPH)
Haley Brouwer, Meghan Henderson, Marissa Sullivan, and Jenna Walker
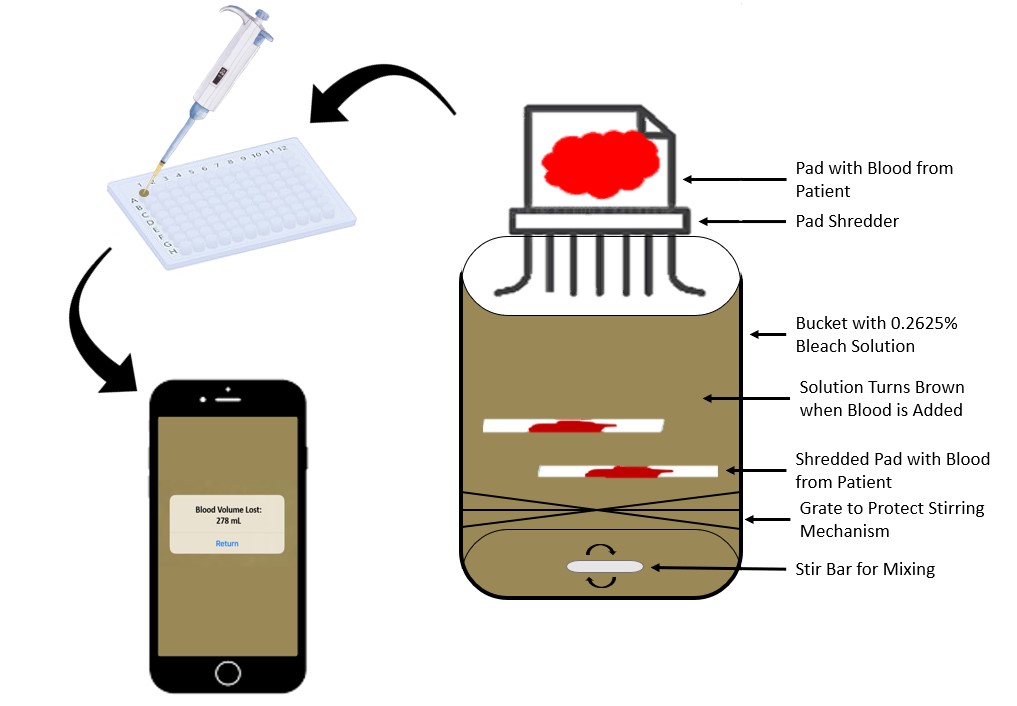 Postpartum hemorrhage (PPH) is excessive and uncontrolled bleeding (over 500 mL) after birth; when not identified and treated, it can lead to large amounts of blood loss, shock, and eventually death. The most common cause of PPH is uterine atony (failure to contract). The United States has the highest maternal mortality rate of any developed nation; an estimated 1 to 5% of American women will experience PPH. Several treatment options exist, but they are only effective if PPH is diagnosed early. The most common method of blood loss estimation is about 41% inaccurate after 1L of blood loss, leading to underestimation and delayed treatment. Therefore, a method is needed to accurately, continuously, and comfortably monitor blood loss over the 24 hours following delivery. This will improve intervention choices, facilitate evidence-based decision making for obstetricians and delivery nurses, and improve mother-baby outcomes. Ultimately, this method will decrease maternal mortality due to postpartum hemorrhage. The solution is designed to be minimally disruptive to the current practice of collecting blood loss in maternity pads. The proposed solution involves the shredding and disposal of blood-soaked maternity pads into a container of 0.2625% bleach made with distilled water, which will induce hemolysis of the red blood cells in the pad. The bleach will convert the released hemoglobin molecules into methemoglobin which has a distinct brown color. A smart phone app can take a photo of the solution, analyze the color, and predict the methemoglobin concentration. Using the total volume of bleach solution, blood, and other bodily fluids in the container as well as the patient’s red blood cell count (which is routinely measured in maternity care), the volume of blood loss can be estimated. This solution is innovative as it can be used bedside, provides near real-time monitoring of blood loss and requires minimal hands on labor from the nursing staff or lab technicians.
Postpartum hemorrhage (PPH) is excessive and uncontrolled bleeding (over 500 mL) after birth; when not identified and treated, it can lead to large amounts of blood loss, shock, and eventually death. The most common cause of PPH is uterine atony (failure to contract). The United States has the highest maternal mortality rate of any developed nation; an estimated 1 to 5% of American women will experience PPH. Several treatment options exist, but they are only effective if PPH is diagnosed early. The most common method of blood loss estimation is about 41% inaccurate after 1L of blood loss, leading to underestimation and delayed treatment. Therefore, a method is needed to accurately, continuously, and comfortably monitor blood loss over the 24 hours following delivery. This will improve intervention choices, facilitate evidence-based decision making for obstetricians and delivery nurses, and improve mother-baby outcomes. Ultimately, this method will decrease maternal mortality due to postpartum hemorrhage. The solution is designed to be minimally disruptive to the current practice of collecting blood loss in maternity pads. The proposed solution involves the shredding and disposal of blood-soaked maternity pads into a container of 0.2625% bleach made with distilled water, which will induce hemolysis of the red blood cells in the pad. The bleach will convert the released hemoglobin molecules into methemoglobin which has a distinct brown color. A smart phone app can take a photo of the solution, analyze the color, and predict the methemoglobin concentration. Using the total volume of bleach solution, blood, and other bodily fluids in the container as well as the patient’s red blood cell count (which is routinely measured in maternity care), the volume of blood loss can be estimated. This solution is innovative as it can be used bedside, provides near real-time monitoring of blood loss and requires minimal hands on labor from the nursing staff or lab technicians.
Wireless Powering of Implantable Devices using Current Clinical Frequency Bands
AJ Anderson, I-Ting Chen, Sahar Ibrahim, Sydney Sofronici, James Wang
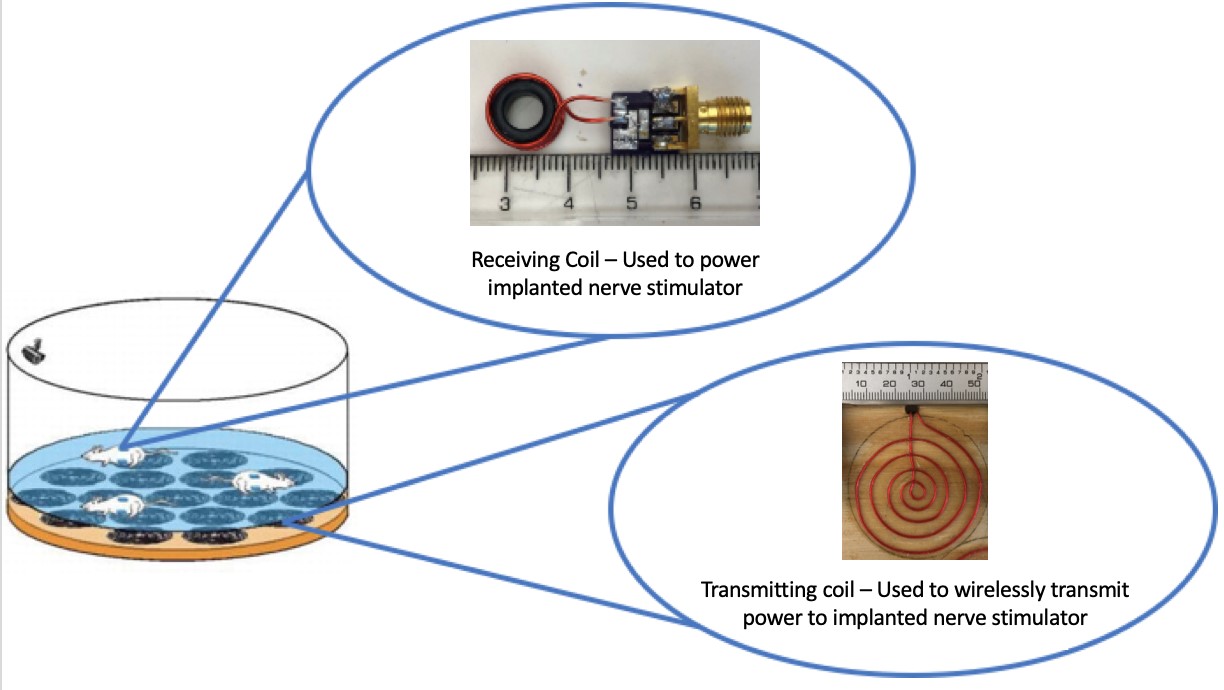 With the $96.6 billion dollar electronic implantable medical device market growing to $143 billion by 2024, there is a magnified need for well-developed wireless powering capabilities. At the Center for Implantable Devices at Purdue University, the current method to wirelessly power a nerve stimulator transfers power using a wireless powering cavity at 340 MHz, and two orthogonal coils to receive power. In order to apply this to more thorough and flexible animal model studies, it is necessary to eliminate the use of the wireless power cavity and decrease the resonant frequency to the clinically accepted frequency of 13.553 to 13.567 MHz frequency band while still providing a uniform magnetic field to the cage. The proposed solution (shown in Figure 1) is a system in which an array of transmitting coils resonates with an implanted receiving coil at 13.56 MHz to power the stimulator. The transmitting coil array is created with eight spiral-shaped coils arranged in a geometry that allows for uniform magnetic field distribution throughout the cage. The receiving coil is a six wind solenoid wrapped around a ferrite core designed to maximize power pickup from the transmitting coil from multiple angles and directions. These solutions in tandem combine multiple approaches to wireless powering to find the optimal system for wireless powering for implantable medical devices.
With the $96.6 billion dollar electronic implantable medical device market growing to $143 billion by 2024, there is a magnified need for well-developed wireless powering capabilities. At the Center for Implantable Devices at Purdue University, the current method to wirelessly power a nerve stimulator transfers power using a wireless powering cavity at 340 MHz, and two orthogonal coils to receive power. In order to apply this to more thorough and flexible animal model studies, it is necessary to eliminate the use of the wireless power cavity and decrease the resonant frequency to the clinically accepted frequency of 13.553 to 13.567 MHz frequency band while still providing a uniform magnetic field to the cage. The proposed solution (shown in Figure 1) is a system in which an array of transmitting coils resonates with an implanted receiving coil at 13.56 MHz to power the stimulator. The transmitting coil array is created with eight spiral-shaped coils arranged in a geometry that allows for uniform magnetic field distribution throughout the cage. The receiving coil is a six wind solenoid wrapped around a ferrite core designed to maximize power pickup from the transmitting coil from multiple angles and directions. These solutions in tandem combine multiple approaches to wireless powering to find the optimal system for wireless powering for implantable medical devices.
WOODS - Wearable Opioid Overdose Detection System
Emma Roberts, Lakmini Wilson, Aamnah Zaim, Brenna Sloan
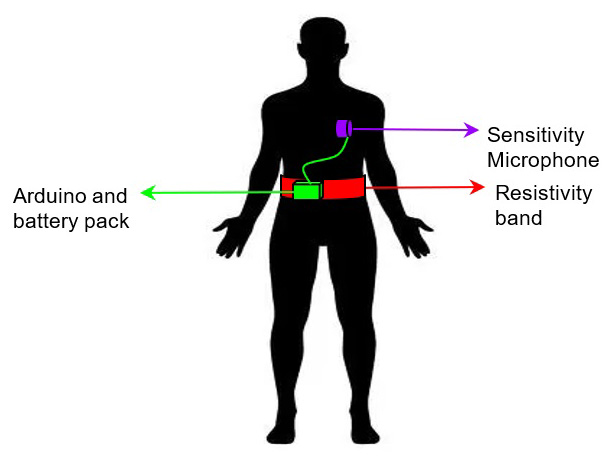 Opioid overdose is an issue that affects millions of individuals worldwide with over 19,000 deaths occurring in the United States from prescribed opioids in 2016. We propose a device that integrates a mechanical respiratory sensor and a sensitivity microphone. This monitoring system can decrease diagnosis time, thus reducing the risk of hypoxia induced brain damage and death. The solution will take input signals from a microphone and a mechanical sensor which detect breathing sounds and chest movements respectively. Each sensor will send the wearer’s respiratory data to a microprocessor for signal processing. The algorithm in the microprocessor will determine if the wearer is experiencing respiratory depression by comparing a recorded respiratory rate to the wearer’s baseline data or falls below 8 breaths per minute (a sign of respiratory depression). This approach is innovative since it uses sensors that focus on both the mechanical and auditory indicators of breathing. Most current approaches rely on one method of measurement which increases the chances of false negatives. Furthermore, this solution uses direct measurements of breath rates instead of measuring indirect biosignals that change during respiratory depression such as blood oxygen levels or pulse which leads to a faster diagnostic time. Hypoxia induced brain damage and death can occur in a matter of minutes; thus, there is a need for a wearable respiratory depression detection system to reduce fatality of opioid overdose in recovering addicts by reducing diagnostic time. Our solution aims to address this need through a diagnosis of respiratory failure in under 15 seconds, so patients can get immediate, life-saving medical attention.
Opioid overdose is an issue that affects millions of individuals worldwide with over 19,000 deaths occurring in the United States from prescribed opioids in 2016. We propose a device that integrates a mechanical respiratory sensor and a sensitivity microphone. This monitoring system can decrease diagnosis time, thus reducing the risk of hypoxia induced brain damage and death. The solution will take input signals from a microphone and a mechanical sensor which detect breathing sounds and chest movements respectively. Each sensor will send the wearer’s respiratory data to a microprocessor for signal processing. The algorithm in the microprocessor will determine if the wearer is experiencing respiratory depression by comparing a recorded respiratory rate to the wearer’s baseline data or falls below 8 breaths per minute (a sign of respiratory depression). This approach is innovative since it uses sensors that focus on both the mechanical and auditory indicators of breathing. Most current approaches rely on one method of measurement which increases the chances of false negatives. Furthermore, this solution uses direct measurements of breath rates instead of measuring indirect biosignals that change during respiratory depression such as blood oxygen levels or pulse which leads to a faster diagnostic time. Hypoxia induced brain damage and death can occur in a matter of minutes; thus, there is a need for a wearable respiratory depression detection system to reduce fatality of opioid overdose in recovering addicts by reducing diagnostic time. Our solution aims to address this need through a diagnosis of respiratory failure in under 15 seconds, so patients can get immediate, life-saving medical attention.
Implantable Drug Delivery System to Counteract Respiratory Arrest in Patients at Risk for Opioid Overdose
Aditya Desai, Erika Dow, Mariah McDonald, Sai Nimmagadda
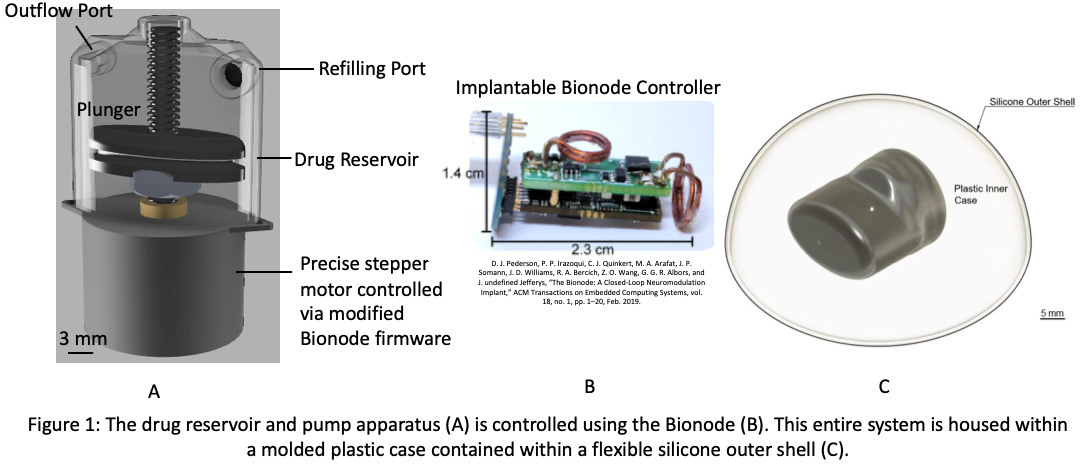 On average, 130 Americans die each day from opioid overdose. Drug users require treatment within one to three hours of overdose for the best chance of survival and are more likely to recover when other people are present to assist in respiratory restoration. Unfortunately, assistance in such situations is difficult to receive as drug users are often alone when they overdose; even if there are other people within reach, they will most likely lack the necessary expertise and access to reversal drugs like naloxone to treat these individuals. Existing methods for counteracting opioid overdose include the administration of naloxone either by nasal spray or injection. Both methods effectively restore respiration but require the presence of a first responder; therefore, the success of dosage administration is largely dependent on 911 response time assuming a witness is present to make the call. Hence, a method which reverses respiratory arrest autonomously and in a timely manner would offer a potential solution to counteract this epidemic. Consequently, there exists a need to develop an implantable drug delivery system with a dosage mechanism that utilizes oxygen levels in the body to detect and counteract respiratory arrest in patients at risk for opioid overdose, specifically patients one to two years out of rehabilitation. These individuals are most at risk for relapse as their tolerance for their usual opioid dosage decreased during rehabilitation; the proposed technology will protect these individuals while they are in this vulnerable stage and potentially reduce the mortality rate associated with opioid addiction. Here, we have developed a closed-loop, implantable device that autonomously administers a single dose of naloxone when the patient first enters respiratory arrest. Once the device detects critically low oxygen levels using an oxygen sensor, the dosage is released into the bloodstream over the course of ninety minutes to sustain the patient for the duration of the overdose until additional medical assistance can be reached. The device is reusable as it has a port that allows for the drug reservoir to be refilled when the patient comes to the emergency room. A flexible, hermetic silicone outer casing is comfortable for the patient while a rigid inner casing protects the pump itself. The pump has been shown to consistently release the required drug volume steadily over the course of ninety minutes while blood pressure measurements in animal studies demonstrate the proper release of drug. As device development continues, human clinical trials are recommended to ensure the design translates to a human patient population. Human clinical trials will provide the required data for achieving regulatory approval to ultimately bring the device to market.
On average, 130 Americans die each day from opioid overdose. Drug users require treatment within one to three hours of overdose for the best chance of survival and are more likely to recover when other people are present to assist in respiratory restoration. Unfortunately, assistance in such situations is difficult to receive as drug users are often alone when they overdose; even if there are other people within reach, they will most likely lack the necessary expertise and access to reversal drugs like naloxone to treat these individuals. Existing methods for counteracting opioid overdose include the administration of naloxone either by nasal spray or injection. Both methods effectively restore respiration but require the presence of a first responder; therefore, the success of dosage administration is largely dependent on 911 response time assuming a witness is present to make the call. Hence, a method which reverses respiratory arrest autonomously and in a timely manner would offer a potential solution to counteract this epidemic. Consequently, there exists a need to develop an implantable drug delivery system with a dosage mechanism that utilizes oxygen levels in the body to detect and counteract respiratory arrest in patients at risk for opioid overdose, specifically patients one to two years out of rehabilitation. These individuals are most at risk for relapse as their tolerance for their usual opioid dosage decreased during rehabilitation; the proposed technology will protect these individuals while they are in this vulnerable stage and potentially reduce the mortality rate associated with opioid addiction. Here, we have developed a closed-loop, implantable device that autonomously administers a single dose of naloxone when the patient first enters respiratory arrest. Once the device detects critically low oxygen levels using an oxygen sensor, the dosage is released into the bloodstream over the course of ninety minutes to sustain the patient for the duration of the overdose until additional medical assistance can be reached. The device is reusable as it has a port that allows for the drug reservoir to be refilled when the patient comes to the emergency room. A flexible, hermetic silicone outer casing is comfortable for the patient while a rigid inner casing protects the pump itself. The pump has been shown to consistently release the required drug volume steadily over the course of ninety minutes while blood pressure measurements in animal studies demonstrate the proper release of drug. As device development continues, human clinical trials are recommended to ensure the design translates to a human patient population. Human clinical trials will provide the required data for achieving regulatory approval to ultimately bring the device to market.
Automated System to Optimize the Duopath Cereus Enterotoxins Test Strip for Use in a Milk Bank Setting
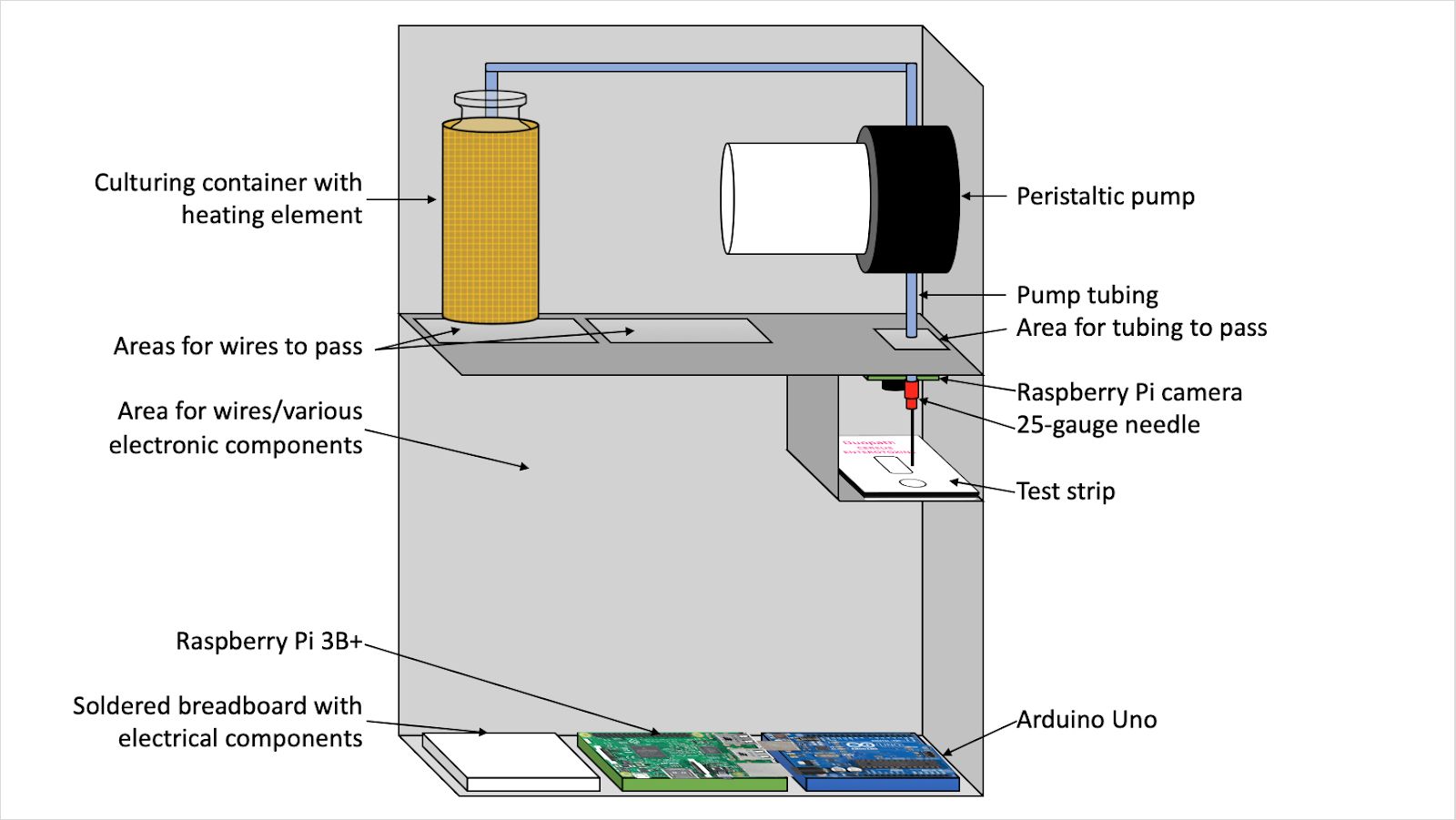 Over 29 million new mothers are unable to breastfeed their babies due to insufficient milk production. Rather than supplement their milk supply with formula, more than 80% of these mothers turn to milk banks to supplement their milk supply to meet the nutritional needs of their babies. Human milk banks collect donated breast milk from donors across the country, pasteurize it, and distribute it to both mothers in need and neonatal intensive care units (NICUs). To pasteurize the breast milk, individual donations ranging in volume from 1.5oz to 10oz are combined into a single 40 oz batch, which is then put through the pasteurization process. The goal of pasteurization is to eliminate any potential pathogens living in the breast milk that could cause sickness or premature spoiling of the milk. However, there are some pathogens that are not always eliminated through pasteurization. One of these pathogens is Bacillus cereus, a heat-resistant bacteria that is commonly associated with food poisoning. Currently, milk banks send samples from each batch to an offsite lab for culturing, which can take upwards of 48 hours to produce results. This process is time consuming and also costly to the milk bank, as they need to pay for transport of the samples as well as the laboratory services. Additionally, milk banks need to be able to test individual donations for B. cereus before pooling the donations, as a single contaminated donation can ruin an entire 40 oz batch of breast milk. Therefore, there is a need for an efficient, safe, and in-house method that milk banks can use to detect B. cereus in individual breast milk donations. There are some new testing methods coming out on the market, but they are targeted towards the laboratory setting and are not accessible to the average milk bank worker who may not have extensive biology and chemistry experiences. One such method is a rapid detection test strip, Duopath Cereus Enterotoxins, that targets two of the toxins produced by B. cereus during culturing. In order for the test strip to be used, the sample must be incubated at 37 ˚C for 18 hours, and 150 μL of the cultured sample needs to be deposited on the test strip, which can be opened no more than two hours prior to use. Our solution is a device that contains and automates the procedure required for the rapid detection test strip in order to make it more accessible to the average milk ban worker. Using a simple disposable pipette, the milk bank worker will deposit 5 mL of breast milk into a culturing container and press a button, upon which the device will culture the sample for 18 hours using a heating element surrounding the culturing container. Once culturing is complete, the device will alert the worker, who will then open and insert a test strip into the device. The device will then automatically deposit the required 150 μL using a small peristaltic pump onto the sample port of the test strip and, using a camera and photo analysis software, will indicate to the worker whether the sample shows contamination. The entire process is contained within a metal housing unit.
Over 29 million new mothers are unable to breastfeed their babies due to insufficient milk production. Rather than supplement their milk supply with formula, more than 80% of these mothers turn to milk banks to supplement their milk supply to meet the nutritional needs of their babies. Human milk banks collect donated breast milk from donors across the country, pasteurize it, and distribute it to both mothers in need and neonatal intensive care units (NICUs). To pasteurize the breast milk, individual donations ranging in volume from 1.5oz to 10oz are combined into a single 40 oz batch, which is then put through the pasteurization process. The goal of pasteurization is to eliminate any potential pathogens living in the breast milk that could cause sickness or premature spoiling of the milk. However, there are some pathogens that are not always eliminated through pasteurization. One of these pathogens is Bacillus cereus, a heat-resistant bacteria that is commonly associated with food poisoning. Currently, milk banks send samples from each batch to an offsite lab for culturing, which can take upwards of 48 hours to produce results. This process is time consuming and also costly to the milk bank, as they need to pay for transport of the samples as well as the laboratory services. Additionally, milk banks need to be able to test individual donations for B. cereus before pooling the donations, as a single contaminated donation can ruin an entire 40 oz batch of breast milk. Therefore, there is a need for an efficient, safe, and in-house method that milk banks can use to detect B. cereus in individual breast milk donations. There are some new testing methods coming out on the market, but they are targeted towards the laboratory setting and are not accessible to the average milk bank worker who may not have extensive biology and chemistry experiences. One such method is a rapid detection test strip, Duopath Cereus Enterotoxins, that targets two of the toxins produced by B. cereus during culturing. In order for the test strip to be used, the sample must be incubated at 37 ˚C for 18 hours, and 150 μL of the cultured sample needs to be deposited on the test strip, which can be opened no more than two hours prior to use. Our solution is a device that contains and automates the procedure required for the rapid detection test strip in order to make it more accessible to the average milk ban worker. Using a simple disposable pipette, the milk bank worker will deposit 5 mL of breast milk into a culturing container and press a button, upon which the device will culture the sample for 18 hours using a heating element surrounding the culturing container. Once culturing is complete, the device will alert the worker, who will then open and insert a test strip into the device. The device will then automatically deposit the required 150 μL using a small peristaltic pump onto the sample port of the test strip and, using a camera and photo analysis software, will indicate to the worker whether the sample shows contamination. The entire process is contained within a metal housing unit.
Low-Cost Motorized Knee Prosthesis for Above Knee Amputees
Ethan Pappas, Rohit Srivastiva, Kassidy Scott, Greer Smith, Connor Harrell
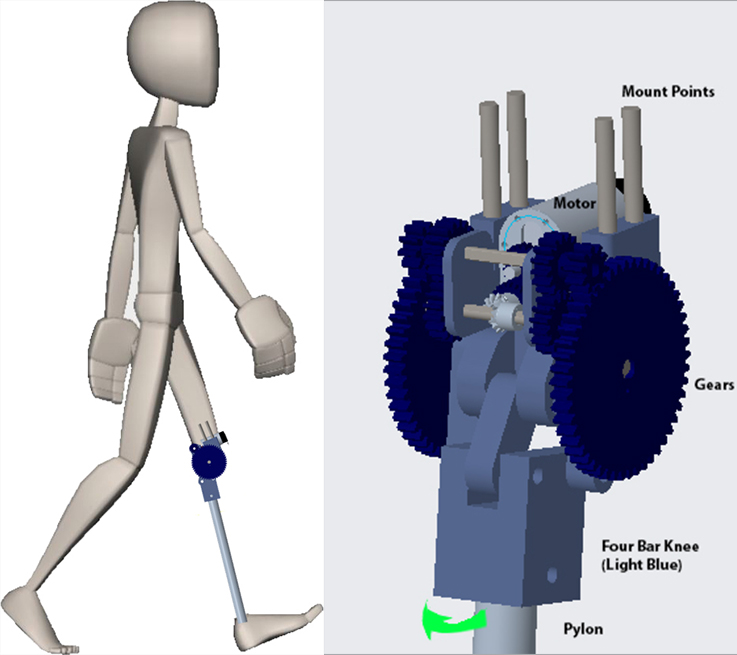 Low income countries, such as Guatemala and Ecuador, have a high prevalence of transfemoral amputees, but existing prostheses are either considerably physically limiting (e.g. a wooden peg) or cripplingly expensive (e.g. a ferrofluid-driven and machine-learning integrated prosthetic). We present a low-cost, motorized, above-knee prosthesis capable of personalizing its gait such that a user can effectively walk at varying speeds or climb stairs. The proposed solution integrates an accelerometer and gyroscope-driven indicator system capable of accurately and reliably identifying an induced step and an open-source microcontroller with adjustable parameters capable of communicating with the accelerometer, gyroscope, and motor to personalize to the user’s gait. This product serves as an affordable, effective alternative to existing products and substantially positively impacts the quality of life of transfemoral amputees.
Low income countries, such as Guatemala and Ecuador, have a high prevalence of transfemoral amputees, but existing prostheses are either considerably physically limiting (e.g. a wooden peg) or cripplingly expensive (e.g. a ferrofluid-driven and machine-learning integrated prosthetic). We present a low-cost, motorized, above-knee prosthesis capable of personalizing its gait such that a user can effectively walk at varying speeds or climb stairs. The proposed solution integrates an accelerometer and gyroscope-driven indicator system capable of accurately and reliably identifying an induced step and an open-source microcontroller with adjustable parameters capable of communicating with the accelerometer, gyroscope, and motor to personalize to the user’s gait. This product serves as an affordable, effective alternative to existing products and substantially positively impacts the quality of life of transfemoral amputees.
Sterile Field Break Detection Using a Capacitive Sensor System
Srinivas Govindan, Apoorva Bhagwat, Ali AbuSaleh, Sawyer Kieffer
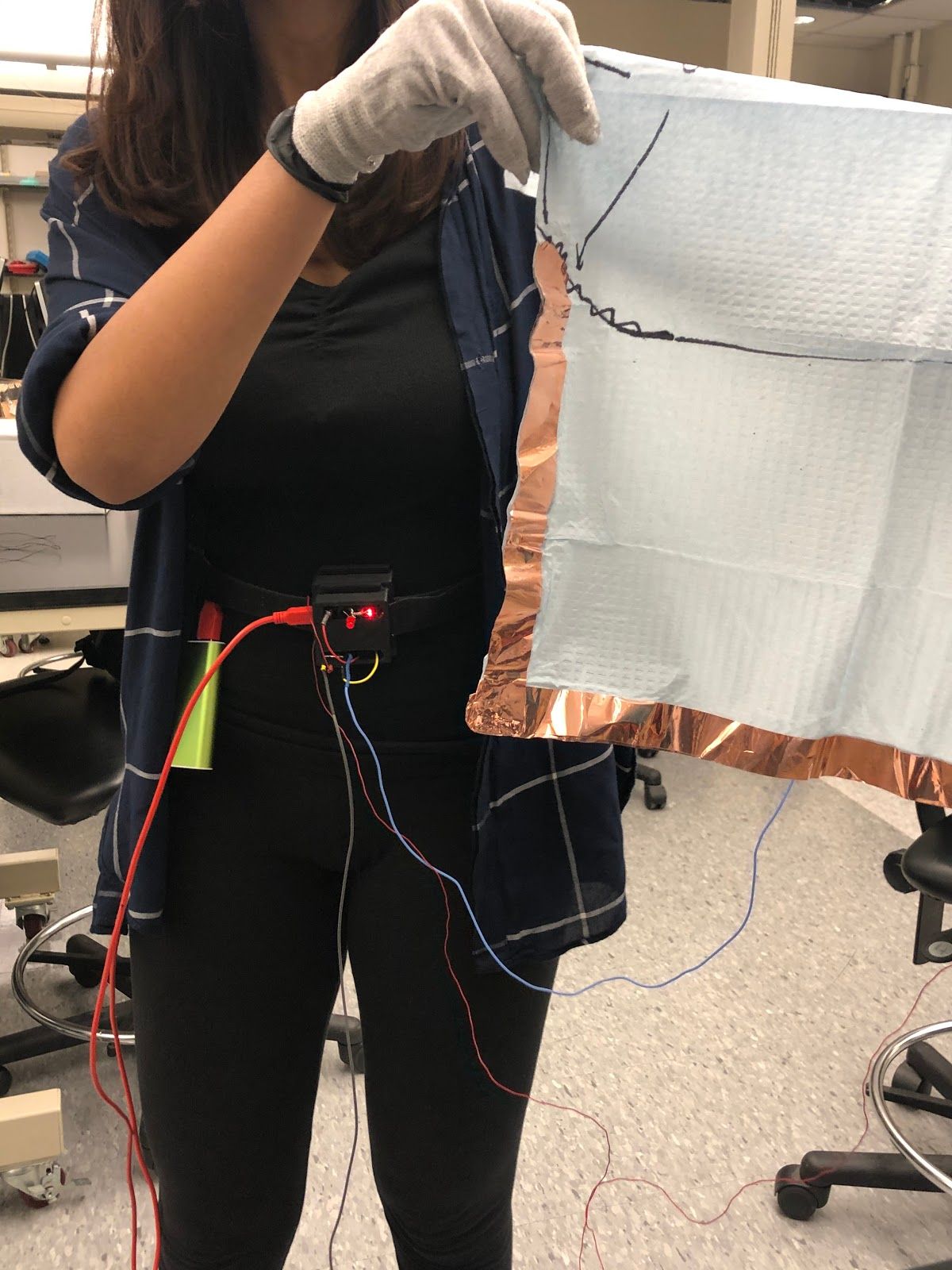 According to the CDC, approximately 70-80% of urinary tract infections can be traced back to indwelling urethral catheters, primarily caused by a break in sterile technique by the healthcare provider during catheter insertion. This indicates a problem with how healthcare providers, particularly nurses, are trained. Currently, there is no way to detect a break in sterile technique in real time during nursing catheter insertion training without faculty supervision. Nicolle et al. have reported that this leads to students attempting to supervise each other, which produces inconsistencies in sterile technique and provides an opportunity for incorrect technique to be carried forward from training. Thus, there is a need for an improved training method for Foley catheter insertion training that accurately detects a break in the sterile field in real time without requiring any additional personnel and allows the user to train more independently, frequently, and consistently, compared to the current training method. Our solution involves creating a capacitive glove sensor system that involves an electrically active glove that can detect contact with conductive surfaces using capacitive sensing. Non-sterile regions that cause most common breaks in the sterile field are covered with conductive paint allowing for the glove to alert the user when it comes into contact with these regions, thus detecting a break in the sterile field in realtime. Upon detection, the system will cause a beeping sound using speakers and the student will be notified of the break. This provides students an opportunity to practice independently and frequently without faculty supervision.
According to the CDC, approximately 70-80% of urinary tract infections can be traced back to indwelling urethral catheters, primarily caused by a break in sterile technique by the healthcare provider during catheter insertion. This indicates a problem with how healthcare providers, particularly nurses, are trained. Currently, there is no way to detect a break in sterile technique in real time during nursing catheter insertion training without faculty supervision. Nicolle et al. have reported that this leads to students attempting to supervise each other, which produces inconsistencies in sterile technique and provides an opportunity for incorrect technique to be carried forward from training. Thus, there is a need for an improved training method for Foley catheter insertion training that accurately detects a break in the sterile field in real time without requiring any additional personnel and allows the user to train more independently, frequently, and consistently, compared to the current training method. Our solution involves creating a capacitive glove sensor system that involves an electrically active glove that can detect contact with conductive surfaces using capacitive sensing. Non-sterile regions that cause most common breaks in the sterile field are covered with conductive paint allowing for the glove to alert the user when it comes into contact with these regions, thus detecting a break in the sterile field in realtime. Upon detection, the system will cause a beeping sound using speakers and the student will be notified of the break. This provides students an opportunity to practice independently and frequently without faculty supervision.
The Bee’s Knee: An Autonomous Knee Brace for ACL Injuries
Jared Badgley, Nick Bluhm, Michael Myers, Ben Walters
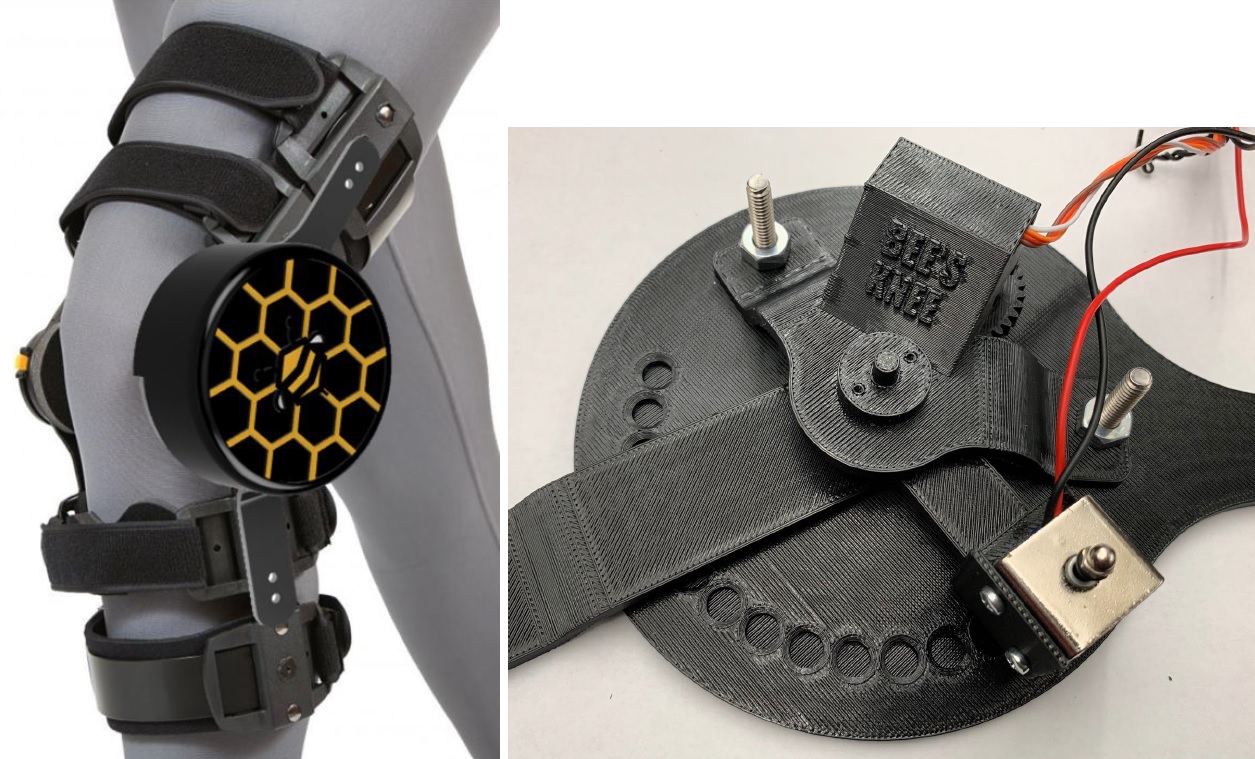 Anterior cruciate ligament (ACL) ruptures are a prevalent injury in young athletes that require a lengthy recovery. The beginning of the recovery process is the most critical time for regaining full range of motion and allowing the ligament to heal correctly. Current braces allow patients to customize the angular range of motion (ROM) for their injured knee, but require manual adjustments. Users cannot be expected to micromanage their braces settings throughout the day and often neglect the recommendations of physical therapists, extending recovery time. Our solution is an autonomous device that monitors the angle and loading of the knee and adjusts the allowable ROM according to thresholds set by physical therapists. These actions are accomplished by utilizing a microcontroller connected to pressure sensors and an angular encoder. These sensors track the loading of the knee and angular position to determine steps taken and fatigue of the joint. The algorithm makes a decision on the allowable ROM based on the calculated fatigue and previous literature data. However, physical therapists are able to make custom adjustments for their patient using a mobile application connected to the device via Bluetooth when the patient is at the physical therapist’s office. The algorithm then sends information to a motor to adjust the location of the locking mechanism, changing the allowable flexion of the knee. The device encourages the user to stretch their leg to full extension when sitting or otherwise at rest, but retain support when the user is walking to prevent heavy loads from being applied to the damaged ACL. Through proper load management, the user will not only experience more freedom in daily activities through automatic angular control but will also experience more effective support of the knee in order to decrease recovery time.
Anterior cruciate ligament (ACL) ruptures are a prevalent injury in young athletes that require a lengthy recovery. The beginning of the recovery process is the most critical time for regaining full range of motion and allowing the ligament to heal correctly. Current braces allow patients to customize the angular range of motion (ROM) for their injured knee, but require manual adjustments. Users cannot be expected to micromanage their braces settings throughout the day and often neglect the recommendations of physical therapists, extending recovery time. Our solution is an autonomous device that monitors the angle and loading of the knee and adjusts the allowable ROM according to thresholds set by physical therapists. These actions are accomplished by utilizing a microcontroller connected to pressure sensors and an angular encoder. These sensors track the loading of the knee and angular position to determine steps taken and fatigue of the joint. The algorithm makes a decision on the allowable ROM based on the calculated fatigue and previous literature data. However, physical therapists are able to make custom adjustments for their patient using a mobile application connected to the device via Bluetooth when the patient is at the physical therapist’s office. The algorithm then sends information to a motor to adjust the location of the locking mechanism, changing the allowable flexion of the knee. The device encourages the user to stretch their leg to full extension when sitting or otherwise at rest, but retain support when the user is walking to prevent heavy loads from being applied to the damaged ACL. Through proper load management, the user will not only experience more freedom in daily activities through automatic angular control but will also experience more effective support of the knee in order to decrease recovery time.
At-home Self-collection of Cells for Cervical Cancer Diagnosis
Catherine Berger, Ana Dias, Minseon Gim, Jonathan Hicks, Eva Yezerets
 Human papilloma virus (HPV) is the cause of 99% of all cervical cancer cases, and 80% of the adult population will contract HPV in their lifetimes. 24.6% of patients who are supposed to get regular cervical cancer testing were not up-to-date on these screenings as of 2016. This is largely due to issues with health education, healthcare costs, discomfort, pain, or trauma from the procedure. While early detection and treatment of precancerous cells is effective and relatively noninvasive, fatal consequences result from failure to test. This is coupled with estimated productivity losses of $2.1 billion due to cervical cancer deaths between the years of 1935 and 2014. Currently, on the market, there is no solution to this problem. Any cervical cancer detection is performed by a doctor, and all related at-home examinations only test for HPV, but not cervical cancer directly. Therefore, there is a need for an at-home screening device specific to cervical cancer that minimizes pain, embarrassment, and discomfort during screening. Any device designed to tackle this issue must collect at least 5,000 cervical cells for standard pathology lab testing. Our device will be used in the cell collection stage of cervical cancer screening, minimizing the negative factors associated with the traditional Pap smear by providing an at-home self-use option for users. After collection, the user’s cervical sample is mailed to a pathology lab for diagnosis, as is common for specimens collected by the majority of medical practitioners. The created device features 7 components: a cell collection brush; a silicone cup which isolates cervical cells; flexible tubing used to sheath a speedometer cable which rotates the brush; an applicator; a turning handle to rotate the speedometer cable; a collection cup; and fixation fluid to prepare cells for a pathology lab. The user inserts the applicator into their vagina and then pushes the handle on the proximal of the speedometer cable, causing the silicone cup on the distal end of the tubing to emerge from the applicator and deploy to isolate the cervix from the vagina. Then, the user can twist the handle to twirl the brush that is touching their cervix, facilitating the collection of cervical cells from the squamocolumnar junction. Next, the user can remove the tubing and device to transfer the cells from the collection brush to the cup. When the collection cup is closed, this will release the fixation fluid, protecting the user from contact with potentially harmful chemicals. Our device is novel in that it removes the need for a medical practitioner in the cervical cell collection process, it differs from current at-home solutions in that it focuses directly on cancerous cells and not on the presence of HPV, and it is composed of devices similar to those the target audience would be comfortable with such as a menstrual cup and a tampon applicator. The device will reach patients who have not previously had sufficient access to Pap smear screenings and aims to reduce the rate of untreated/undiagnosed cervical cancer. It could also be especially useful for patients with greater vulnerability such as transgender men, people who have experienced sexual trauma, or have strict religious beliefs.
Human papilloma virus (HPV) is the cause of 99% of all cervical cancer cases, and 80% of the adult population will contract HPV in their lifetimes. 24.6% of patients who are supposed to get regular cervical cancer testing were not up-to-date on these screenings as of 2016. This is largely due to issues with health education, healthcare costs, discomfort, pain, or trauma from the procedure. While early detection and treatment of precancerous cells is effective and relatively noninvasive, fatal consequences result from failure to test. This is coupled with estimated productivity losses of $2.1 billion due to cervical cancer deaths between the years of 1935 and 2014. Currently, on the market, there is no solution to this problem. Any cervical cancer detection is performed by a doctor, and all related at-home examinations only test for HPV, but not cervical cancer directly. Therefore, there is a need for an at-home screening device specific to cervical cancer that minimizes pain, embarrassment, and discomfort during screening. Any device designed to tackle this issue must collect at least 5,000 cervical cells for standard pathology lab testing. Our device will be used in the cell collection stage of cervical cancer screening, minimizing the negative factors associated with the traditional Pap smear by providing an at-home self-use option for users. After collection, the user’s cervical sample is mailed to a pathology lab for diagnosis, as is common for specimens collected by the majority of medical practitioners. The created device features 7 components: a cell collection brush; a silicone cup which isolates cervical cells; flexible tubing used to sheath a speedometer cable which rotates the brush; an applicator; a turning handle to rotate the speedometer cable; a collection cup; and fixation fluid to prepare cells for a pathology lab. The user inserts the applicator into their vagina and then pushes the handle on the proximal of the speedometer cable, causing the silicone cup on the distal end of the tubing to emerge from the applicator and deploy to isolate the cervix from the vagina. Then, the user can twist the handle to twirl the brush that is touching their cervix, facilitating the collection of cervical cells from the squamocolumnar junction. Next, the user can remove the tubing and device to transfer the cells from the collection brush to the cup. When the collection cup is closed, this will release the fixation fluid, protecting the user from contact with potentially harmful chemicals. Our device is novel in that it removes the need for a medical practitioner in the cervical cell collection process, it differs from current at-home solutions in that it focuses directly on cancerous cells and not on the presence of HPV, and it is composed of devices similar to those the target audience would be comfortable with such as a menstrual cup and a tampon applicator. The device will reach patients who have not previously had sufficient access to Pap smear screenings and aims to reduce the rate of untreated/undiagnosed cervical cancer. It could also be especially useful for patients with greater vulnerability such as transgender men, people who have experienced sexual trauma, or have strict religious beliefs.
OnePill: Redesigning the Prescription Bottle for Pediatric Medication Safety
Prisca Mbachu, Drew Fox, Nick Laten, Megan Hofstetter, Jason Ummel
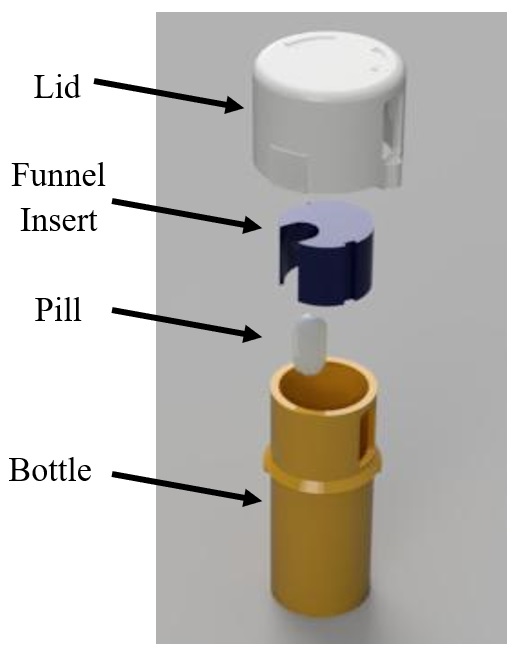 About 95% of emergency room visits among children under the age of 5 are due to a child ingesting medication while unsupervised, and roughly 165 children are brought to the ER each day after taking medication on their own. Considering this, there is a clear need for a device that prevents children from accessing medication at home in order to reduce these incidences of accidental overdose. This device should be able to provide a secure method for storing medicine while still providing adults with limited hand dexterity access to their medication. The proposed design should also be producible in a manner that is cost-competitive with current solutions and compliant with the Poison Prevention Packaging Act set forth by the U.S. Consumer Product Safety Commission. Research indicates that 45-55% of accidental poisonings involve child-resistant packaging, suggesting that the problem is the effectiveness, not availability, of child resistant packaging. This is why we are proposing an alternative to the current prescription bottle design. Our design utilizes a combination of traditional and novel locking methods and incorporates a single-dispense mechanism to limit the amount of medication outflow. The bottle is designed to be filled at the pharmacy then fitted with a permanent lid, so only a single pill can be dispensed from the bottle at once. Despite these additional safety features, our design was constructed to provide minimal resistance to current pharmaceutical workflow, as it is compatible with machines that are used to load and label current prescription bottles. In order to retrieve the medication, multiple operations must be performed. First, the device must be oriented with the chamber in the lid aligned with the slot in the side of the bottle. From this position, one can squeeze the tabs on the lid and rotate the lid clockwise until it no longer turns; this aligns the chamber in the lid with the opening in the funnel insert. The device must then be inverted to allow a single pill to enter the chamber in the lid. While the device is still upside down, one must again squeeze the tabs on the side of the lid and rotate the bottle clockwise; this will realign the chamber in the lid with the slot in the side of the bottle. Aligning these components will dispense a single pill to the user. Due to many cases of medication exposure occurring outside of the clinical setting, pediatric-safe packaging is one of the few physical tools the healthcare system can offer to fight these accidental overdoses. By providing safe, convenient packaging, adults will be disincentivized from storing medication in unsecure secondary packaging, and children will not be able to access the medication even when unsupervised. Successful implementation of our design has the potential to prevent over 60,000 visits to the emergency room, save the medical community over $300 million in operating costs each year in the U.S, and spare children from irreversible damage and possible death due to accidental overdoses.
About 95% of emergency room visits among children under the age of 5 are due to a child ingesting medication while unsupervised, and roughly 165 children are brought to the ER each day after taking medication on their own. Considering this, there is a clear need for a device that prevents children from accessing medication at home in order to reduce these incidences of accidental overdose. This device should be able to provide a secure method for storing medicine while still providing adults with limited hand dexterity access to their medication. The proposed design should also be producible in a manner that is cost-competitive with current solutions and compliant with the Poison Prevention Packaging Act set forth by the U.S. Consumer Product Safety Commission. Research indicates that 45-55% of accidental poisonings involve child-resistant packaging, suggesting that the problem is the effectiveness, not availability, of child resistant packaging. This is why we are proposing an alternative to the current prescription bottle design. Our design utilizes a combination of traditional and novel locking methods and incorporates a single-dispense mechanism to limit the amount of medication outflow. The bottle is designed to be filled at the pharmacy then fitted with a permanent lid, so only a single pill can be dispensed from the bottle at once. Despite these additional safety features, our design was constructed to provide minimal resistance to current pharmaceutical workflow, as it is compatible with machines that are used to load and label current prescription bottles. In order to retrieve the medication, multiple operations must be performed. First, the device must be oriented with the chamber in the lid aligned with the slot in the side of the bottle. From this position, one can squeeze the tabs on the lid and rotate the lid clockwise until it no longer turns; this aligns the chamber in the lid with the opening in the funnel insert. The device must then be inverted to allow a single pill to enter the chamber in the lid. While the device is still upside down, one must again squeeze the tabs on the side of the lid and rotate the bottle clockwise; this will realign the chamber in the lid with the slot in the side of the bottle. Aligning these components will dispense a single pill to the user. Due to many cases of medication exposure occurring outside of the clinical setting, pediatric-safe packaging is one of the few physical tools the healthcare system can offer to fight these accidental overdoses. By providing safe, convenient packaging, adults will be disincentivized from storing medication in unsecure secondary packaging, and children will not be able to access the medication even when unsupervised. Successful implementation of our design has the potential to prevent over 60,000 visits to the emergency room, save the medical community over $300 million in operating costs each year in the U.S, and spare children from irreversible damage and possible death due to accidental overdoses.
Colonoscopic Tool Organization and Retraction Automation
Ryan Harris, Wolfgang Mattke, Michael Williams, Kevin Yeh
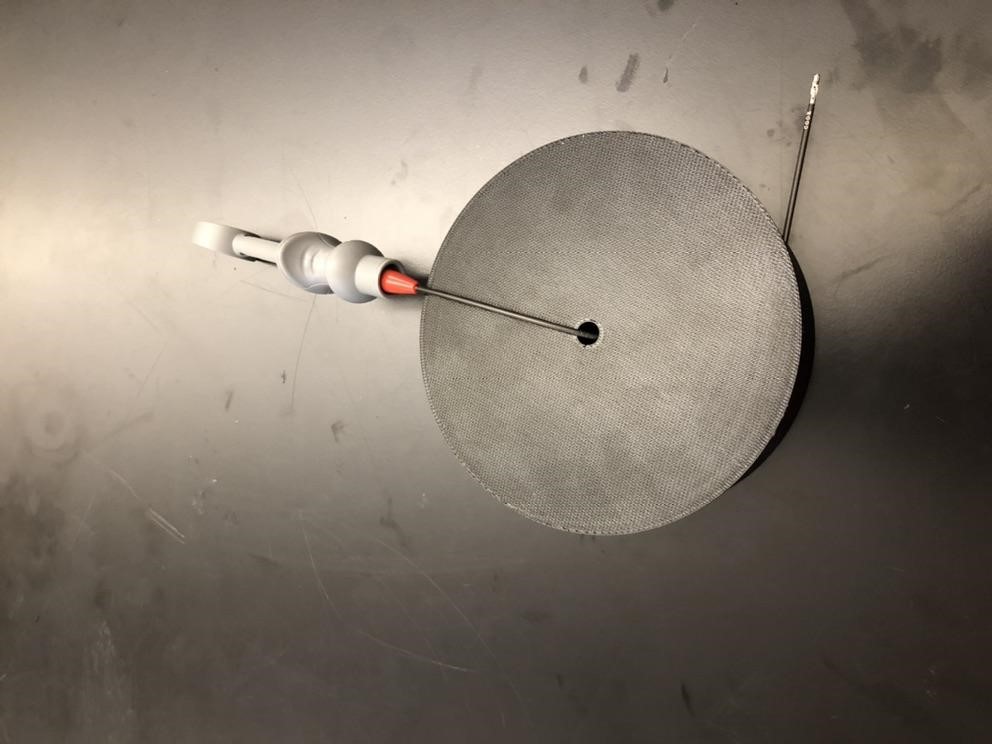 There is a clinical need for an organizational system for colonoscopy tools. The toolage is unwieldy and difficult to retract from the system and store in between usage during polypectomies. In addition, the tools are typically over six feet long and have a strong tendency to uncurl due to the stiffness and can be difficult to control when retracting from the patient without significantly slowing down the process. Our solution is an organizational system that drastically reduces the space each tool takes up and swiftly retracts them into an enclosed container. Our design is innovative due to the novelty of using an automated system for a task that is currently manually done while also eliminating an organizational and logistical problem. Our solution drastically reduces the clutter of colonoscope tools while simplifying a task that will be repeatedly performed during polypectomies. This has the effect of reducing wasted time, wasted colonoscope tools, and simplifying the overall procedure.
There is a clinical need for an organizational system for colonoscopy tools. The toolage is unwieldy and difficult to retract from the system and store in between usage during polypectomies. In addition, the tools are typically over six feet long and have a strong tendency to uncurl due to the stiffness and can be difficult to control when retracting from the patient without significantly slowing down the process. Our solution is an organizational system that drastically reduces the space each tool takes up and swiftly retracts them into an enclosed container. Our design is innovative due to the novelty of using an automated system for a task that is currently manually done while also eliminating an organizational and logistical problem. Our solution drastically reduces the clutter of colonoscope tools while simplifying a task that will be repeatedly performed during polypectomies. This has the effect of reducing wasted time, wasted colonoscope tools, and simplifying the overall procedure.
Automatic Detection of Obstructive Sleep Apnea Using a Single Lead ECG Signal
Maddie Henderson and Nick Chelales
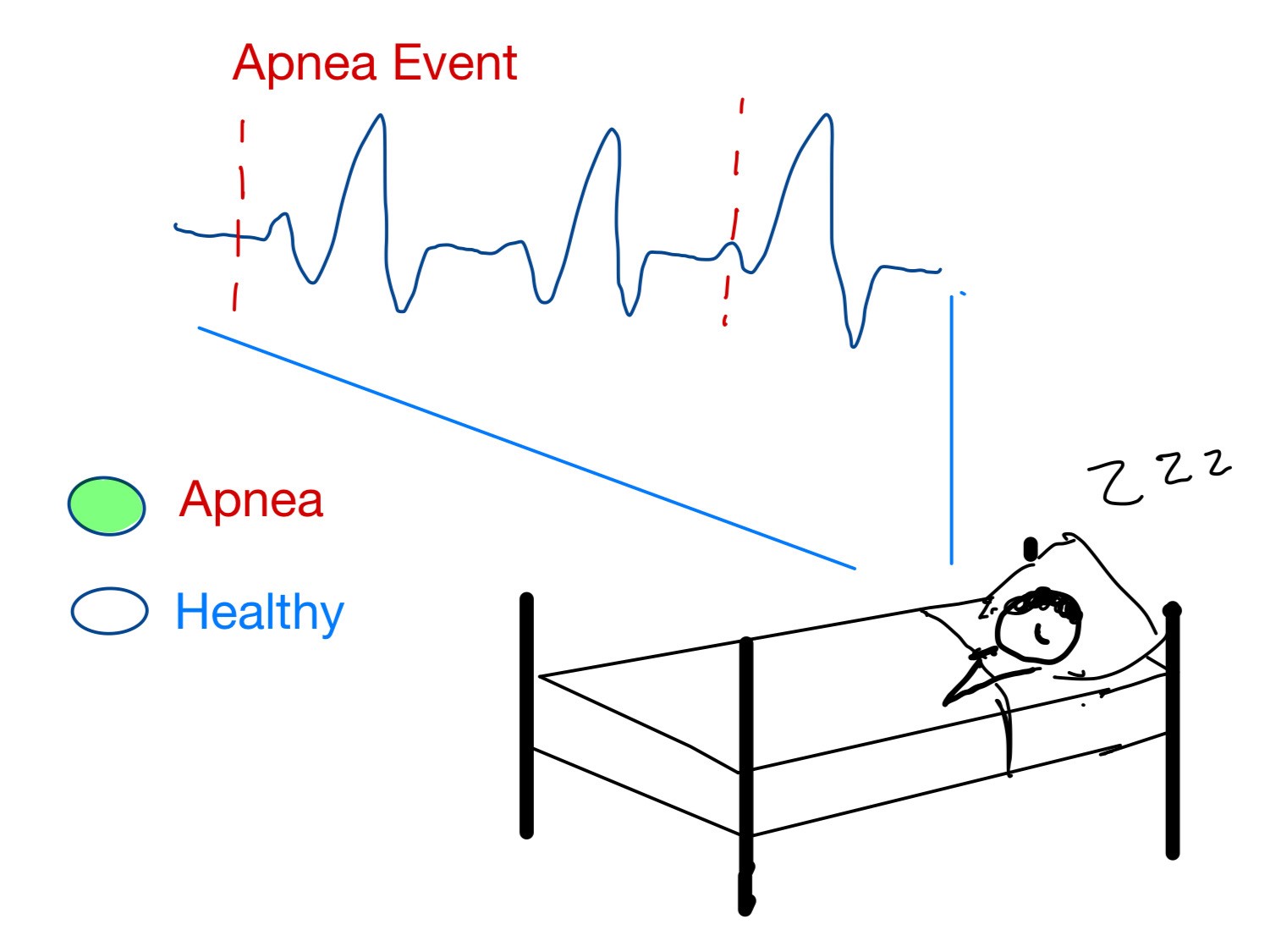 Obstructive sleep apnea (OSA) is a common sleep disorder that can significantly affect human quality of life. Patients who suffer from OSA exhibit symptoms of fatigue, decreased neurocognitive functional capabilities, and increased risk of a stroke or heart complications. Clinically, the most effective method for detecting OSA is through the use of polysomnography where the patient’s heart rhythms (ECG), brain signals (EEG), blood oxygen levels, respiration rate, eye movements (EOG), and leg movements (EMG) are monitored while sleeping in a laboratory or hospital. OSA is commonly undiagnosed due to 1) the difficulty of a patient to recognize their own sleep deficiencies 2) the complicated, expensive, and time-consuming setup of a polysomnography which hinders one’s ability to sleep naturally. There is a clinical need to create a more effective, less expensive, and non-invasive method to detect OSA. In order to overcome the shortcomings of current clinical practices, automatic detection software is currently being developed to detect sleep apnea using only a single lead ECG (electrocardiogram) signal of a patient. The software system consists of three phases: 1) nonlinear feature extraction using discrete wavelet decomposition, 2) algorithm training on various patient data, including selections of best features and classifiers 3) scoring of the apnea-hypopnea index (AHI) using the best algorithm. This solution will allow for at-home signal collection using an already-available wearable ECG monitor instead of a hospital study, so the signals generated are more indicative of the patient’s real sleep. It also provides an automatic pre-diagnosis and AHI score for physicians to review instead of manual signal analysis and scoring. These advantages will save time and money during the diagnostic process for OSA in addition to creating a more effective system with less interruption of natural sleep, leading to better diagnoses and eventually healthier patients.
Obstructive sleep apnea (OSA) is a common sleep disorder that can significantly affect human quality of life. Patients who suffer from OSA exhibit symptoms of fatigue, decreased neurocognitive functional capabilities, and increased risk of a stroke or heart complications. Clinically, the most effective method for detecting OSA is through the use of polysomnography where the patient’s heart rhythms (ECG), brain signals (EEG), blood oxygen levels, respiration rate, eye movements (EOG), and leg movements (EMG) are monitored while sleeping in a laboratory or hospital. OSA is commonly undiagnosed due to 1) the difficulty of a patient to recognize their own sleep deficiencies 2) the complicated, expensive, and time-consuming setup of a polysomnography which hinders one’s ability to sleep naturally. There is a clinical need to create a more effective, less expensive, and non-invasive method to detect OSA. In order to overcome the shortcomings of current clinical practices, automatic detection software is currently being developed to detect sleep apnea using only a single lead ECG (electrocardiogram) signal of a patient. The software system consists of three phases: 1) nonlinear feature extraction using discrete wavelet decomposition, 2) algorithm training on various patient data, including selections of best features and classifiers 3) scoring of the apnea-hypopnea index (AHI) using the best algorithm. This solution will allow for at-home signal collection using an already-available wearable ECG monitor instead of a hospital study, so the signals generated are more indicative of the patient’s real sleep. It also provides an automatic pre-diagnosis and AHI score for physicians to review instead of manual signal analysis and scoring. These advantages will save time and money during the diagnostic process for OSA in addition to creating a more effective system with less interruption of natural sleep, leading to better diagnoses and eventually healthier patients.
