Bryan BalsRose-Hulman Institute of Technology
|
 |
Introduction
Infiltration processing is a relatively common procedure used to make metal-matrix composites. Molten metal is infiltrated into a ceramic preform, thus creating a material with many of the benefits of both metals and ceramics. Such composites have a wide range of applications, from car parts to bicycle frames to satellites. Although much research has been done in determining what liquids can spontaneously infiltrate what preforms, this project focuses on the kinetics of infiltration. There are no simple equations that can apply to all situations due to the infinite complexity in size, shape, and placement of the pores.Project Objectives
- Design an experimental procedure to quickly and easily test the kinetics of infiltration
- Test this setup and compare to accepted data.
Experimental Approach
Tungsten was used as the porous preform in this experiment. A variety of liquids and low-melting metals were tested for spontaneous infiltration. Attempts were also made to infiltrate with various epoxies. This was done to protect the pore integrity during the cutting and polishing process. The pore size and density was also measured in order to analyze the infiltrate rate data. An apparatus was set up to measure the weight gain of the liquid into the porous perform, which was measured against time. Other variables, specifically viscosity and surface force, were measured, as they affect the infiltration rate. By varying viscosity, different sets of data were obtained and compared to current equations.Research Findings
- A glycerin-water mixture was finally chosen as the liquid in this project. This was due to its ability to spontaneously infiltrate at atmospheric conditions and the wide range of viscosity available due to different concentrations.
- A method of infiltrating a sample with M-Bond 610 allowing it to cure, then repeating the process multiple times was set up in order to mount the preforms in epoxy. This is due to the fact that M-Bond 610 shrinks significantly when cured. No better solution was found
- Two different methods of measuring the preform density were used, which gave extremely different results. Counting the pores led to extremely low values, ~35% dense, while submersion tests indicated the value was around 75%. It is suspected that damage to the preform occurred during the cutting and polishing process, and thus resulted in an extremely low number.
- Data was fairly reproducible and generally seemed accurate. However, there were problems with the preform used, namely the fact that it was not uniform and appeared to be damaged after numerous cleansings.
 Fig. 1. Optical microscopy showing the porosity of the preform. The light area is the metal. |
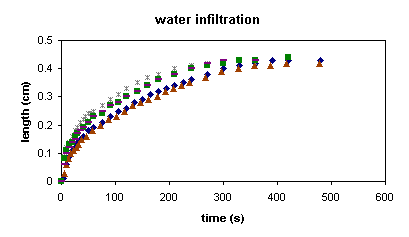 Fig. 2. Plot showing the reproducibility of the data. |
