Treatments following heart attacks could improve with 4D image analysis
In response to injury, the heart undergoes a complex healing process involving structural and functional changes. Purdue University Weldon School of Biomedical Engineering researchers have developed a novel, noninvasive image analysis approach to characterize the mechanical properties of injured mouse hearts. They demonstrated that early changes in cardiac mechanics following a heart attack is predictive of how the heart will repair itself in the long-term. The ability to detect these early changes may allow physicians to identify patients at high-risk of developing heart failure and better manage treatment.
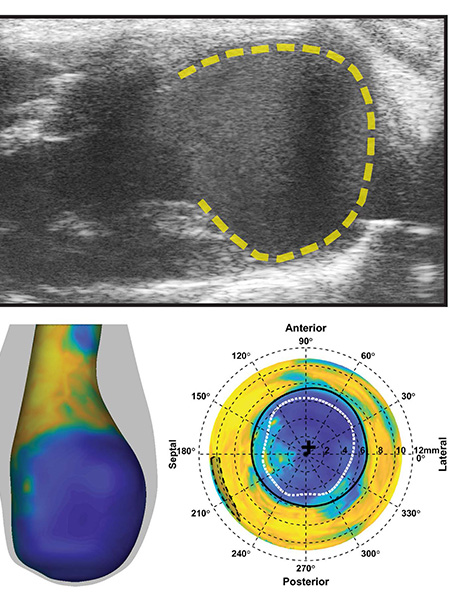
“Our lab has developed a four-dimensional ultrasound technique,” said Arvin Soepriatna, a PhD candidate at the Weldon School of Biomedical Engineering. “We can visualize, in 3-D, how the heart contracts over a representative heart beat and throughout remodeling.” Soepriatna integrated this imaging approach with an image analysis technique developed by research collaborators at Washington University in Saint Louis. The two techniques together enable researchers to noninvasively quantify early, regional damage in the heart and make predictions over time.
“In research, a lot of people still rely on ex vivo mechanical testing,” said Soepriatna. “They take tissue out and test it to get an actual material property assumption. Using our technique, we wanted to see if we could noninvasively characterize mechanics without performing ex vivo organ testing. What we found is that, once we integrated the two techniques together, we can predict how far tissue damage will extend towards as early as day one post-injury.”
The researchers were able to predict which mouse models would go into heart failure and which would have severe damage to the heart by looking at early mechanical changes. In order to test the approach in humans, a method is needed to acquire four-dimensional ultrasound images in humans.
This work is supported by the Hugh W. and Edna M. Donnan Fellowship, the American Heart Association, and the Indiana Clinical and Translational Sciences Institute, funded in part by the National Institutes of Health. The authors wish to acknowledge Drs. John Boyle and Guy Genin from Washington University in Saint Louis and Stavros Thomopoulos from Columbia University (formerly of Washington University) for their collaboration on this research.
ABSTRACT
Three-dimensional myocardial strain correlates with murine left ventricular remodelling severity post-infarction
Arvin H. Soepriatna1, A. Kevin Yeh1, Abigail D. Clifford2, Semih E. Bezci3, Grace D. O’Connell3,4 and Craig J. Goergen1,5
1Weldon School of Biomedical Engineering, Purdue University
2Department of Animal Sciences, Purdue University
3Department of Mechanical Engineering, University of California - Berkeley
4Department of Orthopaedic Surgery, University of California - San Francisco
5Center for Cancer Research, Purdue University
Heart failure continues to be a common and deadly sequela of myocardial infarction (MI). Despite strong evidence suggesting the importance of myocardial mechanics in cardiac remodelling, many MI studies still rely on two-dimensional analyses to estimate global left ventricular (LV) function. Here, we integrated four-dimensional ultrasound with three-dimensional strain mapping to longitudinally characterize LV mechanics within and around infarcts in order to study the post-MI remodelling process. To induce infarcts with varying severities, we separated 15 mice into three equal-sized groups: (i) sham, (ii) 30 min ischaemia–reperfusion, and (iii) permanent ligation of the left coronary artery. Four-dimensional ultrasound from a high-frequency small animal system was used to monitor changes in LV geometry, function and strain over 28 days. We reconstructed three-dimensional myocardial strain maps and showed that strain profiles at the infarct border followed a sigmoidal behaviour. We also identified that mice with mild remodelling had significantly higher strains in the infarcted myocardium than those with severe injury. Finally, we developed a new approach to non-invasively estimate infarct size from strain maps, which correlated well with histological results. Taken together, the presented work provides a thorough approach to quantify regional strain, an important component when assessing post-MI remodelling.
