NIH grant will advance research on how cells generate and use mechanical forces
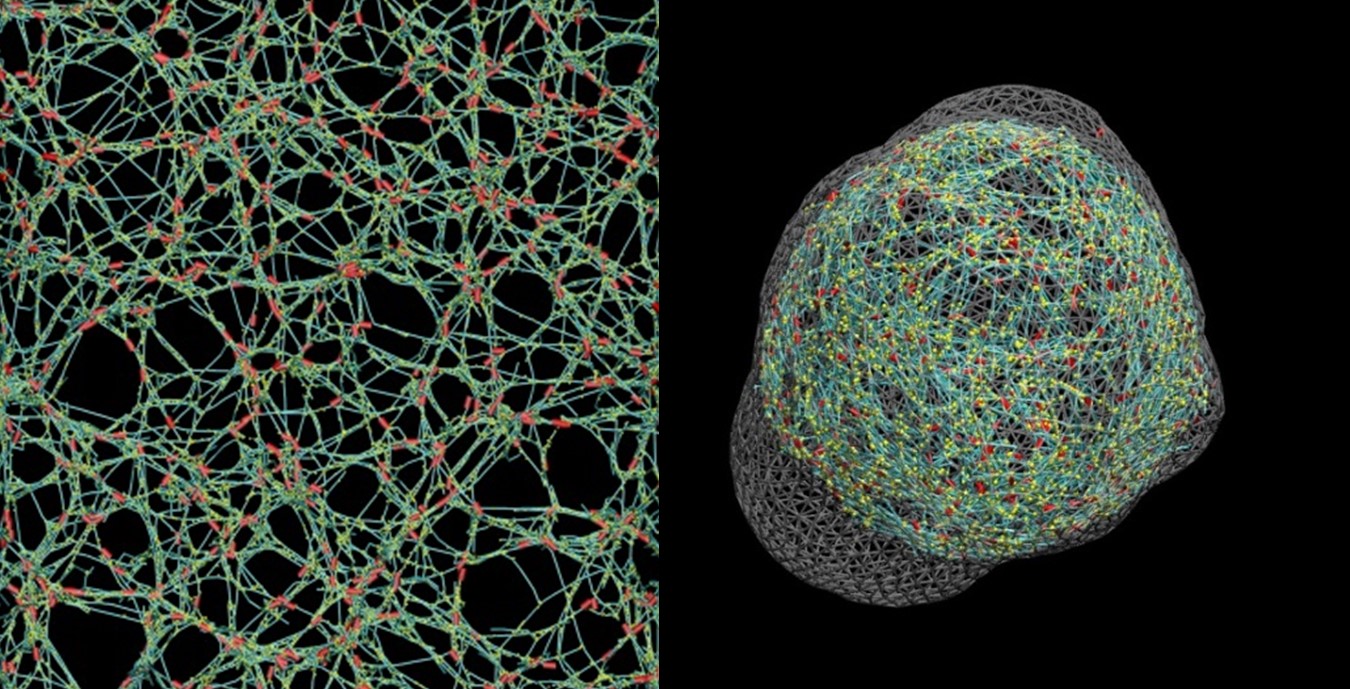 Cells generate mechanical forces to perform many of their biological functions, such as cell division or migration, Kim explained. Cells achieve this primarily via a polymeric structure known as the actin cytoskeleton, in which a protein called myosin binds and pulls actin filaments to generate force as a molecular motor.
Cells generate mechanical forces to perform many of their biological functions, such as cell division or migration, Kim explained. Cells achieve this primarily via a polymeric structure known as the actin cytoskeleton, in which a protein called myosin binds and pulls actin filaments to generate force as a molecular motor.
“We know pretty well how these processes work in muscle cells, but our knowledge about non-muscle cells is way behind,” Kim said. Although the actin filaments are precisely aligned in muscle cells, they are highly disorganized in non-muscle cells. Additionally, actin filaments keep turning over rapidly in non-muscle cells.
These two factors make it very hard to reconstitute the force generation process occurring in cells using in vitro experimental systems. “There is a significant difference between what is happening in the cell and what in vitro experiments have shown using proteins purified from cells, but we can fill the gap between these two with the help of computational models,” he said.
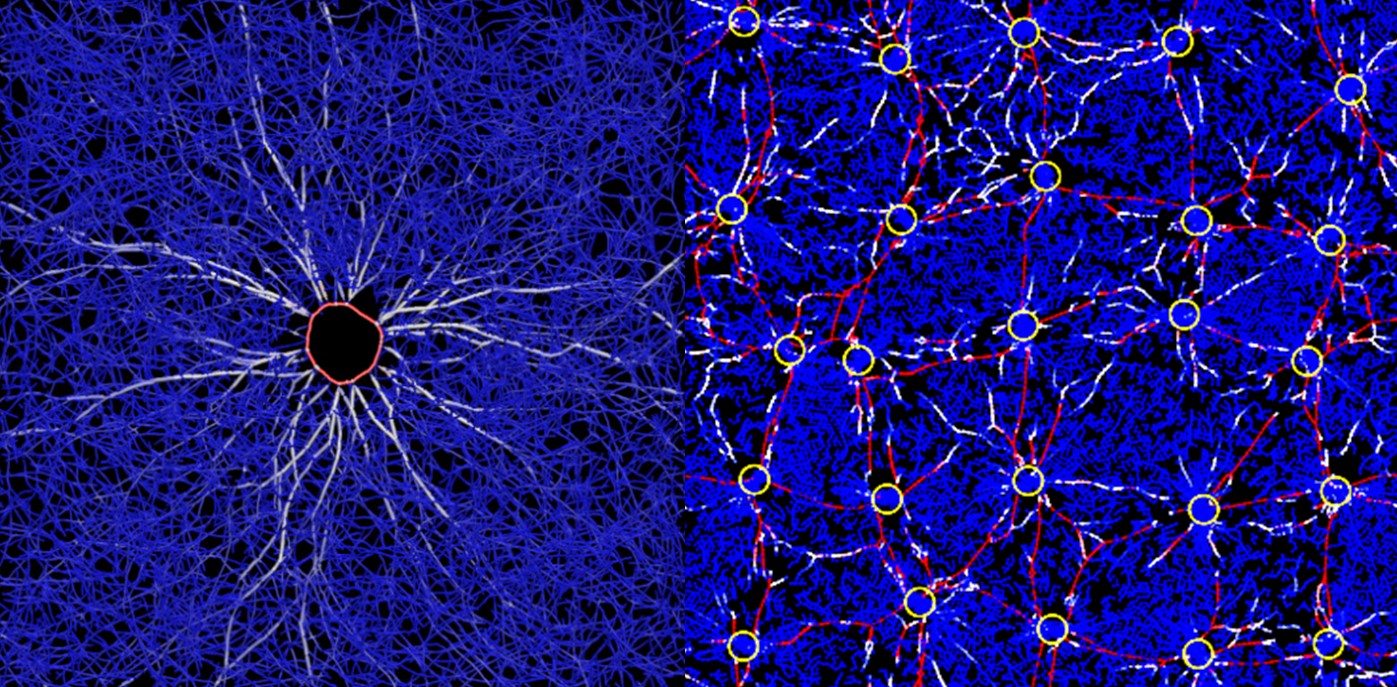 The project will combine multi-scale computational modeling with experimental works using both artificial cells and animal cells. Kim will primarily use a supercomputing environment at Purdue ITaP for the intense computation required for his multi-scale models. The models then will be validated and fine-tuned by results from in vitro experiments in the labs of co-investigators Michael Murrell, assistant professor of biomedical engineering at Yale University, and Ovijit Chaudhuri, assistant professor of mechanical engineering at Stanford University.
The project will combine multi-scale computational modeling with experimental works using both artificial cells and animal cells. Kim will primarily use a supercomputing environment at Purdue ITaP for the intense computation required for his multi-scale models. The models then will be validated and fine-tuned by results from in vitro experiments in the labs of co-investigators Michael Murrell, assistant professor of biomedical engineering at Yale University, and Ovijit Chaudhuri, assistant professor of mechanical engineering at Stanford University.
The first main focus of the project is on how the disorganized actin cytoskeleton generates forces inside a cell, and how those forces change the structure of the cytoskeleton and thus the shape of the cell. Murrell, an expert in experimental mechanics of cellular structures, will lead in vitro studies using reconstituted actin cytoskeleton and artificial cells.
The second main focus will be on how the actin cytoskeleton can remodel the ECM surrounding the cell, and then how the ECM can transmit mechanical forces between cells. Chaudhuri, who studies interactions between cells and ECM, will lead the in vitro work examining these processes using animal cells embedded in three-dimensional ECM.
“Insights about how cells generate and use force will help us to understand various pathophysiological processes, such as cancer metastasis,” Kim commented. Among its roles in metastasis, the actin cytoskeleton drives how cancer cells exert forces and deform their bodies as they migrate through tissues, and how these cells communicate biomechanically via ECM with other types of cells that pave the road for their migration, he said.
Source:
Taeyoon Kim, Ph.D., Assistant Professor, Weldon School of Biomedical Engineering, Purdue University;
kimty@purdue.edu, 765-494-4797, http://engineering.purdue.edu/mct
