Purdue researchers bringing new freeze-drying methods to market
Freeze-drying is as costly a process as it is important. Though it can take days or weeks to properly freeze-dry pharmaceutical products, the process can make many drugs stable enough to be stored and transported without deep-chill freezers. Freeze-drying, or lyophilization, is used in about 25% of new injectable drugs, vaccines and biological products, in addition to certain food products.
The cost of deep-freeze transportation and storage makes some treatments inaccessible in parts of the world. Plus, there's simply not enough lyophilization capacity to make the amount of freeze-dried medications needed. Speeding up the freeze-drying process could save lives around the world.
Purdue University researchers, led by Alina Aleexenko, professor in the School of Aeronautics and Astronautics and Chemical Engineering, have been trying to solve that problem - and they could be close to bringing a solution to market. Alexeenko and her team at LyoHUB, which includes Dimitrios Peroulis, Reilly Professor of Electrical and Computer Engineering, have nearly completed technology that is easy to adapt to existing freeze-drying cabinets and cut the process time in half. A nearly $1 million grant helped them get there.
"Everything about freeze-drying is challenging. It involves the entire textbook of fluid dynamics — multiphase, viscous, porous, compressible and rarefied flows are encountered in various parts of the system. As a fluid dynamicist, I am fascinated by that complexity," she says.
The conditions inside a freeze-dryer are similar to Earth's atmosphere more than 80 kilometers up: It's extremely cold, and the air pressure is extremely low. The usual rules for how fluids behave don't apply anymore. Alexeenko's expertise is in rarefied gas dynamics, a field developed for space exploration, and all her computational analysis and design methods apply directly to lyophilization.
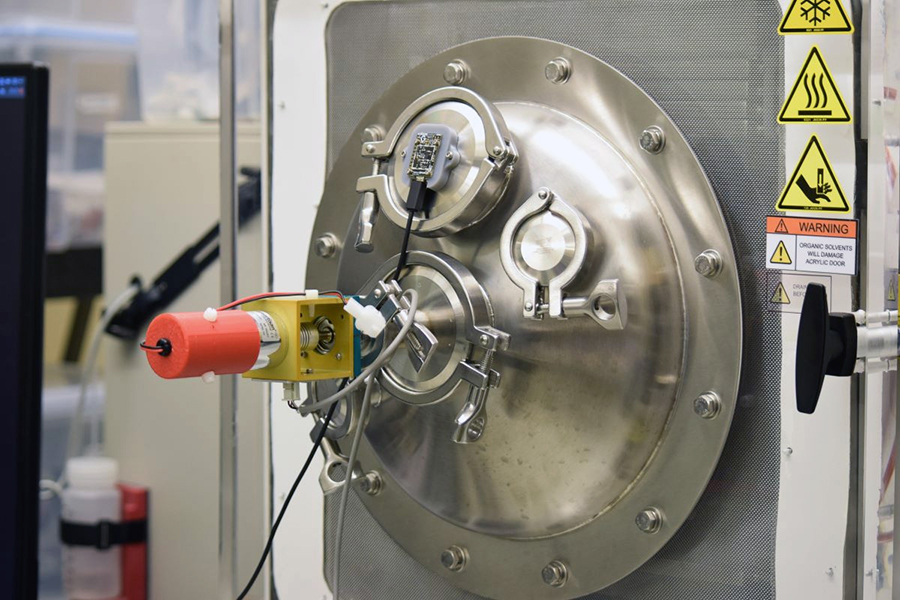
The traditional lyophilization process involves, naturally, freezing, then drying. Once the product has been super-cooled, a little energy is added back in to encourage trapped ice to sublimate - to skip the liquid phase and convert directly from a solid to a gas, allowing it to escape.
But heat doesn’t transfer easily in a near-vacuum. In a lyophilizer, racks of product are touching shelves that are cooled or heated by circulating a fluid like silicone oil through them. Not only is this slow and energy-intensive, it can lead to inconsistent results across large batches.
Alexeenko and her team have been working to improve the use of microwave energy to do the heating portion. Microwaves can travel easily in a vacuum and heat trapped moisture at the molecular level, making the heating portion of the process near-instantaneous. Temperature sensors on the product can be used in a feedback loop to control the heating.

The main difference between these microwaves and the kitchen variety is the frequency: Theirs operate around 18 GHz; conventional microwaves run at 2.45 GHz. This gives a more efficient heat input to ice. Another critical feature of the Purdue technology is producing highly uniform heating. Regular microwave ovens have hot spots and counteract this with a turntable. The team flipped this idea and found a way to rotate the electromagnetic field in random directions, while the biopharmaceutical product remains stationary.
LyoWave Inc., Alexeenko's startup company, is working with Purdue Innovates Office of Technology Commercialization to bring the RF/microwave lyophilization technology to market.
“Freeze-drying increases the shelf-life of our most important and life-saving medicines from a few days to months or even years. That’s obviously a great thing, but the hard truth that pharmaceutical companies have to face is that it’s also slow, risky and expensive,” says LyoWave’s CEO Drew Strongrich, who is an alumnus of Purdue AAE.
“The growing demand for these products combined with limited global production capacity means that we could soon hit a bottleneck,” says Strongrich. “The industry has been looking for a solution to this problem for a long time.”
Their million-dollar grant from NIIMBL came through their Global Health Fund, established by the Bill & Melinda Gates Foundation.
Pharmaceutical companies Merck and IMA Life North America have partnered with Purdue on the project, providing tools to bring it to life. Merck is sharing vaccine samples and developing benchmarks to compare microwave lyophilization with the conventional method. IMA Life will integrate the new technology with the entire manufacturing process for sterile pharmaceuticals and vaccines.
This microwave technology promises to not only provide uniformity to the drug product but also accelerate the freeze-drying process, says Ernesto Renzi, president of sales and marketing at IMA Life.
Karen Plaut, Purdue's executive vice president of research, touts this significant technological advancement as one of the ways the university is able to make a difference.
"Centers like LyoHUB are a great example of how we draw upon Purdue's deep research strengths and state-of-the-art facilities, and leverage the expertise of industry and government to improve our world," says Plaut.
In addition to Alexeenko and Peroulis, the Purdue research team on this project includes:
- Eric Munson, Department of Industrial and Physical Pharmacy
- Vivek Narsimhan, Davidson School of Chemical Engineering
- Qi (Tony) Zhou, Department of Industrial and Physical Pharmacy
