Electrochemical Cycling Dynamics of Polycrystalline LiCoO2 Films
First-year MSE graduate student Tony Chung (R. Edwin Garcia, graduate advisor) has explored the underlying physics that govern a recently developed characterization technique, electrochemical strain microscopy (ESM), that utilizes the strong coupling between ionic current and anisotropic volumetric chemical expansion of lithium-ion electrode materials to dynamically probe the sub-one-hundred-nm interfacial kinetic intercalation properties. The performed analysis demonstrates that the local intercalation dynamics within a LiCoO2 thin film and the implications that such kinetics have on the macroscopic power density performance of currently used Lithium-ion batteries.
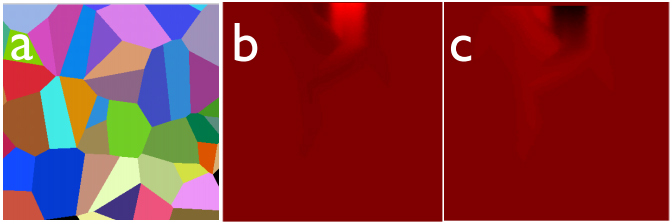
Lithium concentration distribution in crystallographically anisotropic LiCoO2 polycrystalline thin-film cathode layer (a) at different ESM overpotential values; (b) positive (battery discharge) and (c) negative (battery recharge) values. As the overpotential cycles, the lithium diffusion in and out of the film following a tortuous path, induces a volumetric chemical expansion/shrinkage that is captured by the ESM probe.
