Abstract:
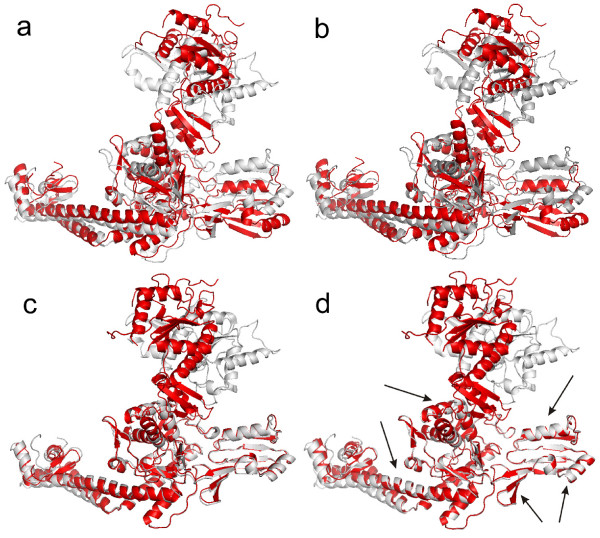
Background: The conventional superposition methods use an ordinary least squares (LS) fit for structural comparison of two different conformations of the same protein. The main problem of the LS fit that it is sensitive to outliers, i.e. large displacements of the original structures superimposed.
Results: To overcome this problem, we present a new algorithm to overlap two protein conformations by their atomic coordinates using a robust statistics technique: least median of
squares (LMS). In order to effectively approximate the LMS optimization, the forward search technique is utilized. Our algorithm can automatically detect and superimpose the rigid core
regions of two conformations with small or large displacements. In contrast, most existing superposition techniques strongly depend on the initial LS estimating for the entire atom sets of
proteins. They may fail on structural superposition of two conformations with large displacements. The presented LMS fit can be considered as an alternative and complementary tool for structural
superposition.
Conclusion: The proposed algorithm is robust and does not require any prior knowledge of the flexible regions. Furthermore, we show that the LMS fit can be extended to multiple level
superposition between two conformations with several rigid domains. Our fit tool has produced successful superpositions when applied to proteins for which two conformations are known. The
binary executable program for Windows platform, tested examples, and database are available from https://engineering.purdue.edu/PRECISE/LMSfit.
Link to paper:  http://www.biomedcentral.com/1471-2105/10/29
Download paper here