Catalyst
To get a healing agent to polymerize may take a catalyst. It depends on the type of healing agent. Some healing agents can polymerize when in contact with air, while other healing agents require a chemical catalyst to polymerize.
If a monomer - catalyst system is going to be used, it is very important to pick a system with the right properties. Specifically the type of catalysis that will occur. A system that has been proven to work well is the Ring Opening Metathesis Polymerization (ROMP) process. There are a few key advantages of ROMP, but two are very important. The first is that the ROMP process is not self terminating. The second is that it is very tolerant to widely variable concentrations of catalyst in the monomer.
The first advantage, that it is not self terminating, is important since the released healing agent may only come in contact with a few catalyst particles. Without the ROMP process, the healing agent in contact with the catalyst would polymerize, form a solid shell around the catalyst and effectively cut-off the remainder of the healing agent. On the other hand, with ROMP, the metathesis process allows the canalized polymer to act as a catalyst and effectively prevent the isolation of the catalyst.
The second advantage, that is is tolerant to variable concentrations, is also very important. Just like the explanation above, the healing agent may only come in contact with a few catalyst particles. Or, it may come in contact with a large clump of catalyst. There is no way to control the effective ratio of healing agent to catalyst. So you need a process that can handle small and large catalyst ratios.
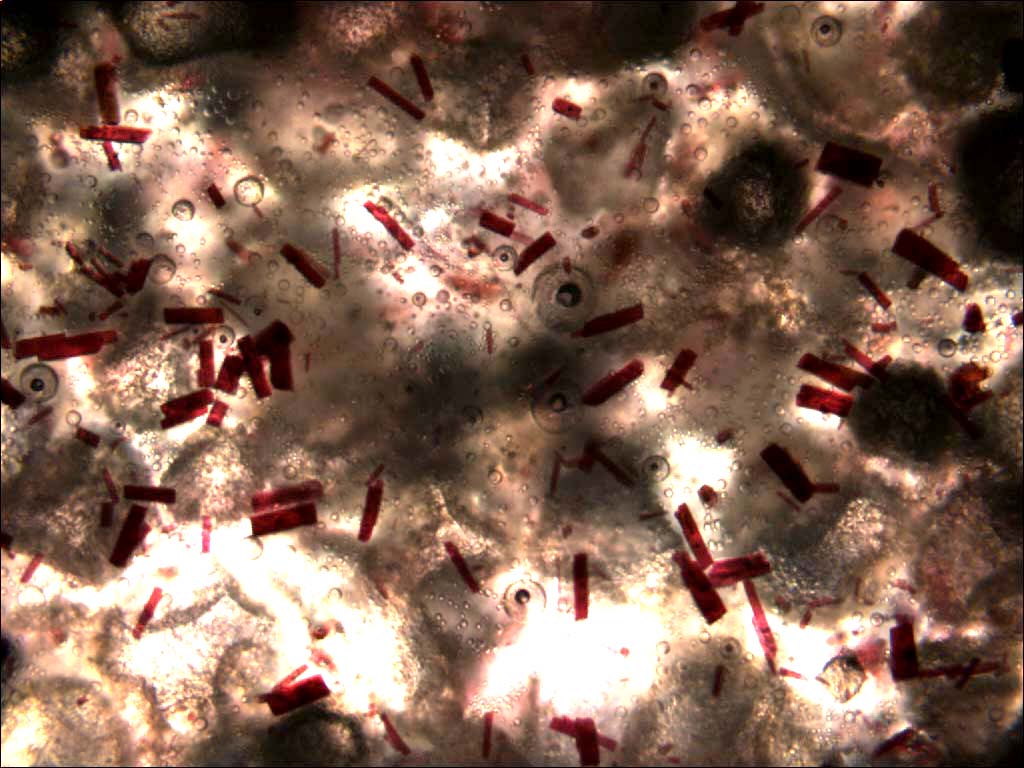
This image shows the epoxy host material with catalyst particles distributed throughout it. Any random crack that propagates through this material will likely expose some of the catalyst. In other words, the crack planes will have some catalyst material on them. When a microcapsule full of healing agent ruptures and releases the healing agent, the healing agent will flow onto the crack plane and come in contact with the catalyst.

The catalyst particles need to dissolve into the healing agent rather quickly. And depending on how the catalyst is processed different morphologies are obtainable. When the catalyst is purchased it could come in many different forms. This is an example of how the catalyst comes from the supplier.

This picture shows the catalyst after it has been processed to increase the surface area to improve dissolution. Without modifying the catalyst in this way the dissolution is too slow, resulting in very little polymerization of the healing agent before it has evaporated or left the crack plane.
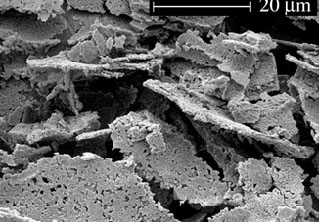
This is the same catalyst material, but it has been processed again to further increase the surface area. This results in a very fast dissolving catalyst that remains stable in the host material. This material can be ground to make it even finer (and dissolve quicker), however, there is a point where the stability of the catalyst is compromised when it is mixed in the host material. The morphology shown here was found to be one of the best compromises between fast dissolution and stability.
