Fermentation and Cell Culture
Fermentation and Cell Culture


Fermentation. The facilities in LORRE are able to carry out aerobic, microaerobic, and anaerobic fermentations using bacteria, yeast, and fungi, and cell culture of immortalized cells to form organoids. We have facilities to conduct fermentations and cell culture, as well as analysis of the products from microwell plates, shake flasks, instrumented 1 L and 10 L fermenters. When combined with our analytical and bioseparations capabilities, these bioreactor capabilities allow students and researchers to conduct fermentations, monitor and analyze fermentation media for expressed bioproducts and proteins. Subsequently the products are purified and completely characterized. These studies, together, are used to develop and validate process models for predicting bioreactor kinetics, and production of proteins and biochemicals. We also utilize catalysts ranging from enzyme mimetics to acids to zeolites for direct transformation of agricultural feedstocks to value-added molecules.
Cell Culture. LORRE also has facilities and capabilities to perform 2D and 3D mammalian cell cultures both in flasks and 50 mL bioreactors. When combined with LORRE fermentation capabilities, the immortalized cell cultures allow LORRE to perform infection detection studies in living organoids. Currently, LORRE performs research with human colorectal adenocarcinoma (DLD-1) and Crandell-Rees Feline Kidney Cell (CRFK) cell lines. Those are cultivated in liquid medium for mammalian cells (RPMI, developed by Roswell Park Memorial Institute), and organoids and micro-clusters are then infected by bacterial and viral pathogens for both infection detection studies and drug resistance studies. For that, LORRE has a laboratory space dedicated exclusively for cell culture research, as mammalian cell cultures require Biosafety Level 2 certification and personnel extensive training, as well as an approved IBC protocol.

Renewable feedstocks for biocatalytic and thermocatalytic reactions include both Newtonian and non-Newtonian fluids consisting of non-pretreated corn stover or other lignocellulosic feedstocks. In the case of lignocellulosic biomass solids, we have carried out biomass liquefaction to achieve concentrations of up to 300 g/L at atmospheric pressure, resulting in low shear stress (low viscosity) lignocellulosic fermentation media. These slurries are used as biomass feedstocks for enzyme catalyzed formation of oligosaccharides and monosaccharides, leaving behind lignin and lignocellulosic solids. The slurries also serve as fermentation feedstocks and as starting reactants for high temperature catalytic transformations.
Microorganisms. A range of bacterial and fungal strains are available for research in the topics of energy, bioproducts, food pathogens, and growing organoids (tissue culture). Fungal strains include Aspergillus and Trichoderma species, including A. niger A12 and 3T5B8, as well as T. reesei. Bacteria include Salmonella Enteritidis, E. coli 0157B6-914 with shiga toxin genes detected, and an E. coli 0157 GFP strain (from Amanda Deering Lab) and Listeria innocua and Listeria monocytogenes. Researchers in LORRE also have access to other microbial collections including the USDA ARS Culture Collection (approximately 100,000 strains) and ATCC (approximately 18,000 strains of bacteria, 3,000 animal viruses, and 1,000 plant viruses).
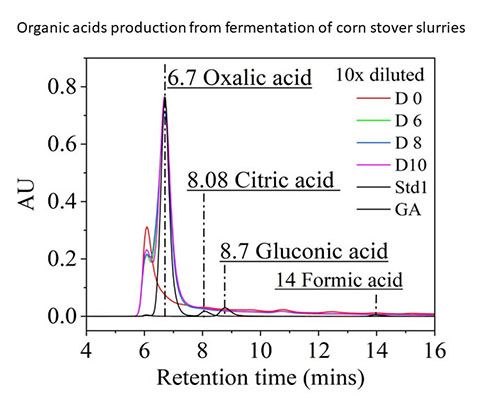
The fermentation of biomass slurry to oxalic acid is an example where a combination of enzyme biocatalysis, biomass liquefaction, and an aerated A. niger fermentation results in a bioproduct that could contribute to decarbonization. Lignocellulose sequesters atmospheric CO2 by virtue of plant growth. The resulting lignocellulose, when liquefied into a lignocellulosic slurry in a fed-batch reactor using a small amount of enzyme, is suitable as a fermentation substrate for A. niger. The citric acid cycle of A. niger can be redirected to form oxalic acid, an acid that can sequester a large fraction of the carbon captured in cellulose in its carbon backbone. When the oxalic acid is used to formulate cement (for preparation) of concrete, the Ca++ or other bivalent cations strongly bind the oxalic acid as an oxalate. This sequesters the carbons in the oxalic acid in the concrete and in effect retain the CO2 captured by the cellulose from which the oxalic acid is formed. A carbon-negative construction material results. This is an example how different bioprocessing capabilities are combined to achieve an over-arching goal, i.e., research on pathways to decarbonization.
