Portable Hydrogen Generation
Motivation
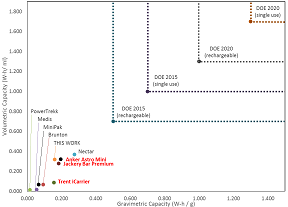
- Determine cost, safety, energy density, and overall feasibility of using ammonia borane in portable power generation applications
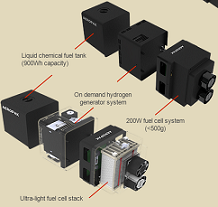
- Develop low-cost, portable systems that can deliver on-demand hydrogen to drive a fuel cell as a direct alternative to batteries
Materials and Techniques
- Ammonia borane (AB) hydrolysis (~9 wt.% hydrogen)
- Reaction initiated with water addition
- Suppresses toxic borazines vapors formed when AB is thermally decomposed
- Challenge in eliminating the ammonia gas produced (compatibility with a proton exchange membrane fuel cell)
- Characterization of catalyzed hydrolysis reaction (see: Ammonia Borane &: Sodium Borohydride)
- Proton exchange resin used as a catalyst
- Comparisons to traditional metal catalyst
- Material Processing
- Platinum black and activated carbon mixed via ball milling
- Amberlyst – 15: ball milled separately to a similar particle size

SEM images of catalysts used, from left to right, Amberlyst-15 (as-received), Amberlyst-15 (ball-milled), and 20% Platinum / 80% Carbon
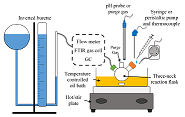
Experimental Setup
- Test the aspects of decomposition reaction that relate to portable operation with a PEM fuel cell
- Control of the water delivery, and reaction environment
- Measurements of temperature, pH, gas production rate, and gas purity
Experimental Results
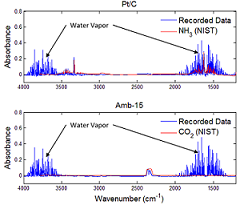
- Gas Purity & Kinetics Studies:
- Objective: determine activation energies and gas compatibility with PEM fuel cells
- Gas chromatography used for hydrogen concentration
- FTIR used to identify contaminate species
- Amberlyst-15 eliminates ammonia production from AB hydrolysis and reduces activation energy by factor of ~5 compared to Pt/C catalyst
Accomplishments/Highlights
- Decomposed ammonia borane via catalyzed hydrolysis
- Tested gas purity and compatibility with PEM-fuel cells
- Characterized hydrolysis reaction kinetics of AB using various catalysts
Multimedia
Hydrolysis of AB with Amberlyst-15. Yield: 95% of theoretical, Reaction duration: 10 seconds, Exotherm: 5°C
Hydrolysis of AB with Pt/C. Yield: 85% of theoretical, Reaction duration: 3 minutes, Exotherm: 20°C
People
PI
Researchers