Hydrogen Adsorption Project
Overview

Zinc-based (left) and Copper-based (right) MOFs
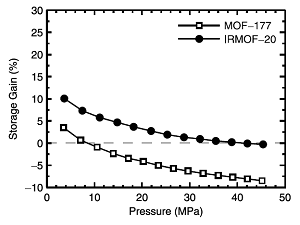
Volumetric hydrogen storage gain relative to compressed gas for MOF-177 and IRMOF-20 at 298 K
The overall objective of this project was to evaluate the hydrogen adsorption characteristics of adsorbent materials at ambient temperatures and high pressures. Adsorbent materials react with hydrogen gas via a physisorption process with the highest hydrogen adsorptions obtained at low pressures, typically under 50 bar and at low temperatures, typically between 77 and 150 K. The materials studied were:
- AX-21 (activated carbon)
- KUA-5 (activated carbon)
- MSC-30 (activated carbon)
- ZTC (zeolite-templated carbon)
- HCP (hyper-crosslinked polymer)
- MOF-177 (metal organic framework)
- IRMOF-20 (metal organic framework)
- MIL-53 (metal organic framework)
- ZIF-8 (metal organic framework)
- Cu3(btc)2 (metal organic framework)
Building upon recent experimental results, hydrogen adsorbent materials were evaluated at room temperature but at pressures significantly higher than those typically associated with these materials. This work used the custom made high pressure Sievert system developed in a previous colloboration with GM.
Accomplishments
- Measured total storage increase for a range of adsorbent materials at room temperature and high pressure
- Identified which materials had net positive storage under normal conditions
- Compared the effect of pore size and open metal sites on hydrogen storage capacity
People
Program Manager
Dr. Mei Cai, General Motors R&D Center
PI
Researchers
, Postdoc (Now at Sandia)