
Viktor J. Cybulskis, P.E.
Graduate Research Assistant
Co-advised by Professors Fabio Ribeiro and W. Nicholas DelgassBackground
Education
- Purdue University, B.S. Chemical Engineering (2005)
- Purdue University, Ph.D. Chemical Engineering (2016)
Experience
- TPC Group, Production Engineer (2009 – 2011)
- LyondellBasell, Research Engineer (2007 – 2009)
- LyondellBasell, Production Engineer (2005 – 2007)
Certifications
- Licensed Professional Engineer in Indiana, PE11400325
Awards
- 37th Michigan Catalysis Society Spring Symposium Oral Presentation Award (2016)
- Purdue Chemical Engineering Graduate Symposium First Place Oral Presentation (2015)
- Purdue Chemical Engineering Excellence in Safety Award (2015)
- Purdue Presidential Safety Award (2015)
- National School on Neutron and X-Ray Scattering Group Presentation Winner (2014)
- Catalysis Club of Chicago Spring Symposium Best Student Poster Award (2013)
- Eastman Chemical Company Travel Award (2013)
- Purdue University Community Service Learning Project Grant (2012)
- Eagle Scout (2000)
Project Description
The Surface Chemical Origins of Catalytic Activity by Supported Metals for Water-Gas Shift and Alkane Dehydrogenation Reactions
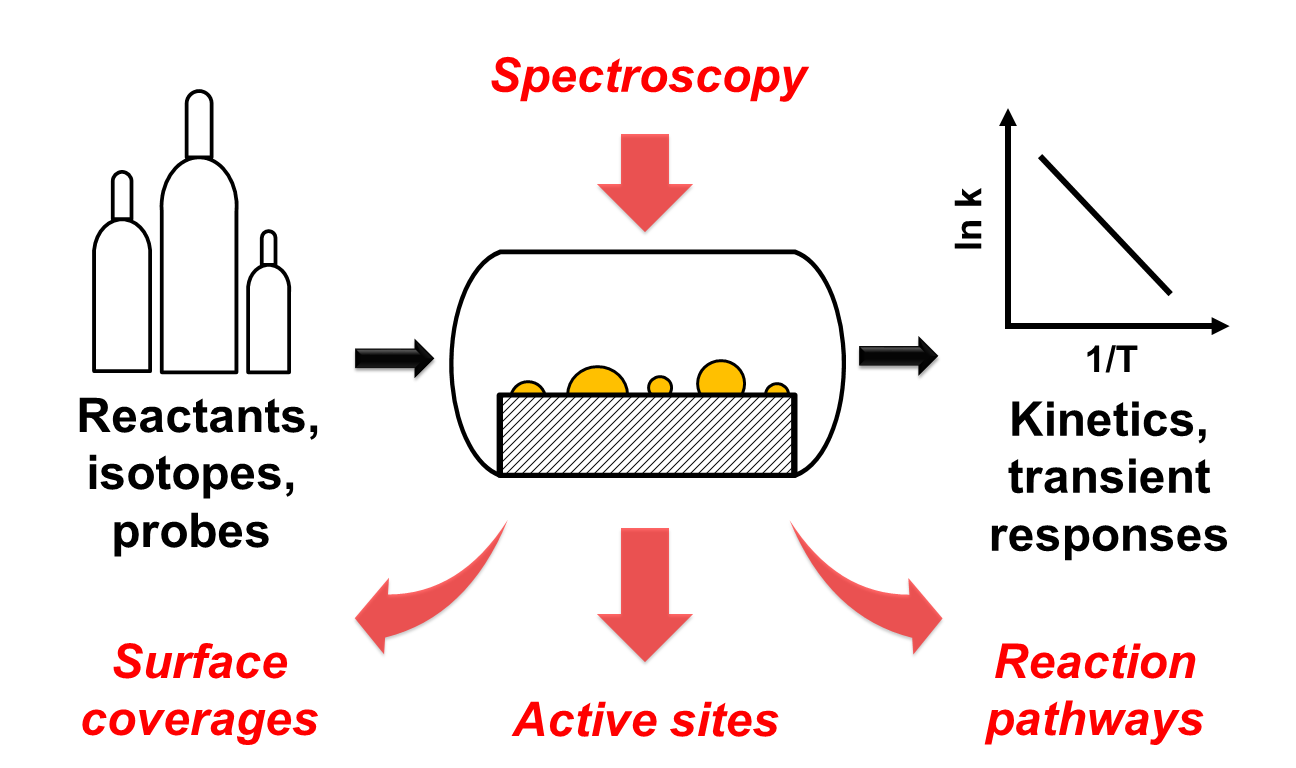
Transition metal catalysts are ubiquitous for industrial chemical reactions such as water-gas shift (WGS), CO oxidation, naphtha isomerization, and alkane dehydrogenation. However, a primary obstacle in optimizing these heterogeneous systems is that the dominant reaction pathways, identities of the active sites, and roles of the metal and support phases are often not well understood. Many of the challenges associated with the design and operation of catalysts to achieve enhanced turnover frequencies (TOF) and product selectivities can only be addressed at the molecular level. Thus, the primary objective of this research is to obtain a fundamental understanding of the surface chemistries that occur on supported metals, such as Pt, Zn, and Ga, by examining specific catalytic phenomena under reaction conditions.
Because of its relative simplicity, the WGS reaction is an ideal system for studying catalytic chemistry on supported noble metal surfaces. Experimental evidence indicates that WGS catalysts which exhibit the highest turnover frequencies operate in a bi-functional manner with contributions from both the dispersed metal as well as the support in activating CO and H2O, respectively. The WGS TOF per exposed surface metal on supported Pt catalysts can vary by a factor of 100 depending upon the metal oxide support. In this work, operando infrared spectroscopy (IR) and transient kinetics with CO/13CO and H2O/D2O isotope switches are used to examine the carbon and hydrogen reactive pools on Pt/Al2O3, Pt/ZrO2, Pt/CeO2, and Na-Pt/Al2O3 while correlating catalyst performance with surface features related to CO and H2O activation. The presence of a hydrogen/deuterium (H/D) kinetic isotope effect (KIE) along with time-resolved IR spectra of transient surface species, reveals that O-H bond breaking is involved in the rate-determining step on alkali-free Pt catalysts. The addition of Na to these Pt-containing materials leads to the formation of new H2O activation sites that modify the rate-determining O-H dissociation step such that nearly all of the CO adsorbed on the Pt surface is able to participate in the reaction. When normalized by the true number of catalytic sites rather than simply by the exposed metal surface, the WGS TOFs are comparable for Na-free Pt and Na-doped Pt samples.
Supported metal catalysts are also vital in a number of hydrocarbon processes. Recent surges in natural gas production from gas-containing shale formations throughout the United States have spurred interest in catalytic solutions to convert these lower alkanes directly into value-added chemicals and fuels. Previous alkane dehydrogenation technologies based on PtSn/Al2O3 and CrOx/Al2O3 catalysts offer improvements over thermal cracking for the production light alkenes, but issues regarding product selectivity and catalyst stability limit their widespread application. Isolated metal centers, which are known to catalyze structure-insensitive reactions such as dehydrogenation, were examined for ethane (EDH) and propane dehydrogenation (PDH). Ga3+ single-sites on SiO2 demonstrated over 95% C3H6 selectivity for 12 h during PDH at 550°C, but suffered from low TOF. SiO2-supported Pt, which exhibits a strong affinity to paraffinic C-H bonds, was shown to become nearly 100% selective to C2H4 during EDH at 600°C upon incorporation of Zn to form a Pt1Zn1 intermetallic alloy. An integrated approach using density functional theory (DFT) methods, kinetics, and in situ X-ray spectroscopy, revealed that the role of Zn is two-fold: (i) to isolate Pt surface sites and suppress C-C bond cleavage, and (ii) to modify the Pt surface electronic structure and promote the EDH TOF per exposed surface Pt by a factor of ten for PtZn/SiO2 compared to Pt/SiO2. The experimental validation of DFT predictions suggests that the measurement of energy transfer between the unoccupied and occupied Pt valence d-shells, rather than the d-band center alone, may provide insight toward predicting the reactivity of metals and, ultimately, toward guiding catalyst design.
Publications and Patents
- Viktor J. Cybulskis, Jun Wang, Jorge H. Pazmiño, Fabio H. Ribeiro, W. Nicholas Delgass, Isotopic Transient Studies of Sodium Promotion of Pt/Al2O3 for the Water-Gas Shift Reaction, Journal of Catalysis, (2016) accepted.
- Viktor J. Cybulskis, Fabio H. Ribeiro, Rajamani Gounder, Using a Hands-On Hydrogen Peroxide Decomposition Activity to Teach Catalysis Concepts to K-12 Students, Journal of Chemical Education, (2016) accepted.
- Viktor J. Cybulskis P.E., Andrew D. Smeltz, Yury Zvinevich, Rajamani Gounder, W. Nicholas Delgass, Fabio H. Ribeiro, Learning the Fundamentals of Kinetics and Reaction Engineering with the Catalytic Oxidation of Methane, Chemical Engineering Education, (2016) accepted. | Supplementary Information
- “Steam Cracking Process”, V.J. Cybulskis and K.M. Webber, Pending U.S. Patent Application US-20110073524-A1 (2011) filed on September 25 (2009).


