
Michael Detwiler
Graduate Research Assistant
Co-advised by Professors Fabio Ribeiro and W. Nicholas DelgassBackground
Education
Youngstown State University, B. Eng. Chemical Engineering (2010)
Purdue University Ph.D. Chemical Engineering (2010 - present)
Experience
Bechtel Marine Propulsion Corporation, Engineering Intern (Summer 2010 and Summer 2009)
Youngstown State University, Peer Tutor (Aug. 2007 - May 2010)
University of South Carolina, Dept. of Chem. Engr. Summer Undergraduate Research Assistant (Summer 2008)
Awards
Outstanding Research Poster Presentation Award – Catalysis Club of Chicago Spring Symposium (2013)
Estus H. and Vashti L. Magoon Award for Excellence in Teaching – Purdue University (2012)
Professional Promise Award – AIChE Pittsburgh Section (2010)
Engineering Student of the Year – Youngstown State University (2010)
About Me
Mike is from the Youngstown, Ohio area. In his free time, Mike enjoys tennis, backpacking, weightlifting, reading, and computers.
Project Description
Catalytic conversion of biomass to liquid fuels and valuable chemicals has emerged as a promising method for renewable fuel and chemical production. Understanding how the structure of a catalyst is related to catalyst performance under reaction conditions is vital for catalyst design, however, biomass upgrading reactions are often performed under harsh conditions involving high temperatures or liquid phase reactants. Learning about catalyst surfaces under these conditions is difficult. Mike's research involves the synthesis, characterization, and catalytic kinetic evaluation of model catalytic systems in closely controlled ultra-high vacuum environments, and the extrapolation of these data to real systems. Mike is involved in several projects:
1. Formic acid decomposition on metal single crystals.
Conversion of biomass-derived sugars to liquid fuels requires a huge input of hydrogen in order to facilitate the removal of oxygen. During this process, formic acid is produced as a byproduct, which can decompose catalytically to hydrogen and carbon dioxide, reducing the required external hydrogen input requirement (Green Chem., 12 (2010) 1493-1513). Mike studies the decomposition of formic acid on different metal single crystal surfaces in order to determine the structure sensitivity of this reaction so that the microkinetic model developed by our collaborators may be validated. Combined with characterization techniques such as X-ray photoelectron spectroscopy, Auger electron spectroscopy, and Fourier transform infrared spectroscopy, Mike and his collaborators will be able to elucidate the formic acid decomposition reaction mechanism on different metal surfaces, validate the microkinetic model, and use the model to design catalysts that maximize hydrogen yield.
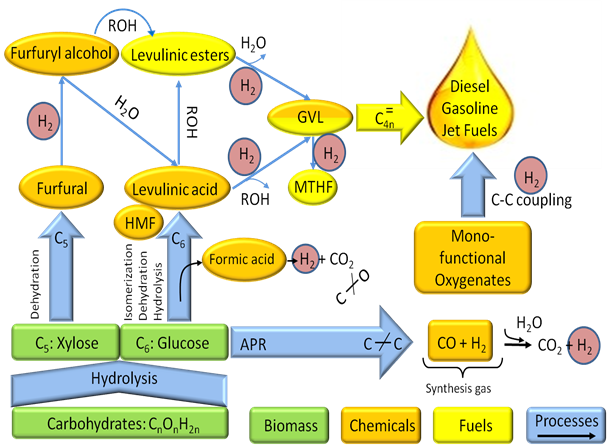
Formic acid decomposition is one reaction of interest to the biomass upgrading reaction network being studied by the Institute for Atom-Efficient Chemical Transformations.
2. Synthesis and characterization of novel catalysts prepared by atomic layer deposition.
Atomic Layer Deposition (ALD) is a self-limiting technique for the deposition of material from the gas phase onto a solid substrate. Though ALD has long been used for the production of microelectronics, it has only recently gained attention for catalyst synthesis. Namely, ALD has been used to form precisely-controlled bimetallic nanoparticles (Nat. Commun. 5:3264 doi: 10.1038/ncomms4264 (2014)), and ALD oxide films known as "overcoats" which prevent supported metal nanoparticles from deactivating by coking and sintering under harsh processing conditions (Science, 335 (2012) 1205-1208).
Chemical information on the as-synthesized ALD catalysts is acquired from X-ray photoelectron spectroscopy and high resolution electron energy loss spectroscopy; we complement these techniques with true atomic-scale scanning tunneling microscopy for structural information.
3. Characterization of powdered bimetallic catalysts for hydrodeoxygenation of lignin model compounds.
Lignin comprises 15 - 30% of total biomass by weight on average, and is a promising feedstock for the production of specialty chemicals and fuels. Our group has been studying the hydrodeoxygenation of lignin model compounds by a variety of bimetallic catalysts. Unlike the above projects which involve model catalysts supported on single crystals, these bimetallic catalysts are prepared by traditional methods. As a result, the complex catalyst structures contain of a variety of phases, oxidation states, and physical structures. Mike has used X-ray photoelectron spectroscopy to characterize these catalysts, while others in the catalysis group have performed reaction kinetics and other complementary characterization techniques including X-ray absorption and scanning transmission electron microscopy in order to build a complete picture of the relationship between catalyst structure and function.
Publications, Presentations, and Patents
Publications
- Michael D. Detwiler , Amir Gharachorlou , Lukas Mayr , Xiang-Kui Gu , Bin Liu , Jeffrey Greeley , W. Nicholas Delgass , Fabio H Ribeiro , and Dmitry Y. Zemlyanov, Reaction of Trimethylaluminum with Water on Pt(111) and Pd(111) from 10-5 to 10-1 Millibar, J. Phys. Chem. C (4)
- J. Rebelli, M. Detwiler, S. Ma, C. T. Williams, J. R. Monnier, Synthesis and characterization of Au-Pd/SiO2 bimetallic catalysts prepared by electroless deposition, J. Catalysis. 270(2) (2010) 224-233.
Presentations
- M. D. Detwiler, A. Gharachorlou, A. V. Nartova, D. Y. Zemlyanov, W. N. Delgass, F. H. Ribeiro, Gas phase formic acid decomposition on platinum single crystal and polycrystalline surfaces: Kinetics and characterization, Poster, Catalysis Club of Chicago Spring Symposium, 2013.
- B. K. Vlaiku, M. D. Detwiler, J. J. Sirko, and A. G. Shaffer, Promoting alternative energy education in middle school science using an algae growth experiment, Oral Presentation, AIChE Annual Student Meeting, 2009.


