Chemical Hydride Hydrolysis
Experimental setup used for chemical hydride hydrolysis. Various instruments can be placed between the reaction vessel and the gas burette, including a gas flow meter (shown), a sampling bulb to allow for collection of samples for gas chromatography, or Dräger tubes for low level detection (<1 ppm) of contaminants.
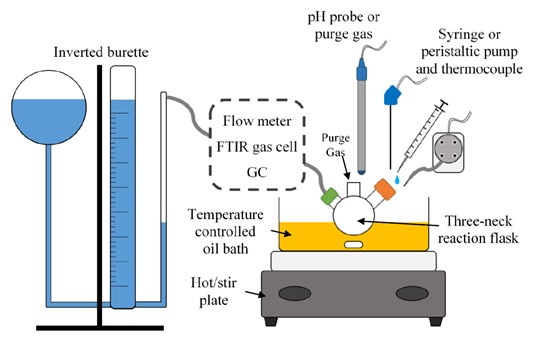 Render of experiment setup.
Render of experiment setup.
 Actual experiment setup.
Actual experiment setup.

Sodium Borohydride
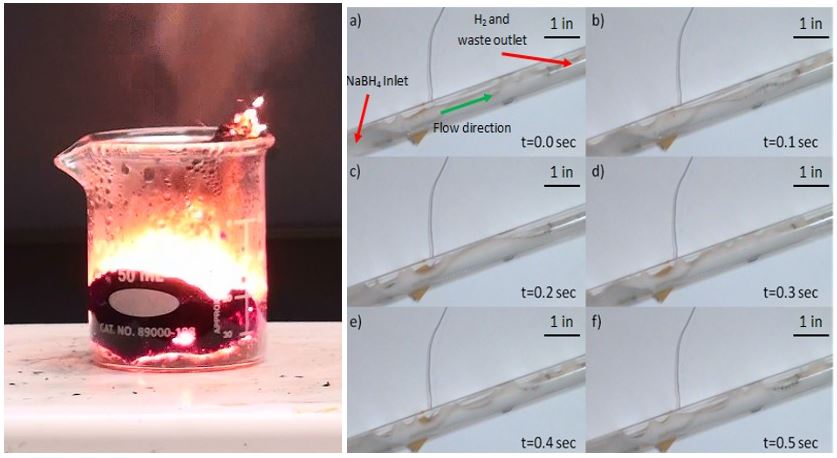 Decomposition reaction of sodium borohydride with low-cost catalysts.
Decomposition reaction of sodium borohydride with low-cost catalysts.
In this Office of Naval Research (ONR) funded project, we focused on developing volumetrically efficient systems for the catalytic decomposition of sodium borohydride. The research involves all aspects of a fuel cell driven power unit, from low-cost catalysts to volumetrically efficient sodium borohydride conditioning and delivery systems.
Among our accomplishments, our team demonstrated a novel cobalt based catalyst. The work focused on solution combustion synthesis (SCS) for rapidly synthesizing a high-purity, nano-foam cobalt oxide powder. This powder is converted in-situ to a magnetic, active catalyst. The magnetic nature of the active catalyst allows for implementation of magnetic immobilization for use in a hydrogen generation system for fuel cell power.

Ammonia Borane
Ammonia borane is a solid state hydrogen storage material that provides a lightweight, low cost, and portable source of energy. Our work with the Office of Naval Research is motivated by the need to provide dismounted infantry with a cost and weight effective power source to replace the heavy batteries currently being carried by soldiers during missions.



Magnesium Hydride Storage
Automotive hydrogen storage systems require a significant increase in the volumetric storage density of hydrogen to provide the necessary range for ordinary use. Solid storage can provide significant increases in volumetric storage capacity of hydrogen at ambient temperatures. In this multi-year collaboration with General Motors, we used TiCrMn as a representative material to investigate:
- Thermal transport restrictions in activated metal hydride powder beds
- Additives to enhance thermal conductivity
- Interactions between thermal and kinetic limitations during tank fueling cycle
- Model development of hydride bed transport phenomena
- Heat exchanger design for metal hydride systems


The overall objective of the proposed program is to develop a magnesium hydride based storage system with rapid kinetics and high storage capacity. The Purdue effort is dedicated to the characterization of the heat and mass transport processes in a representative packed hydride bed.
Magnesium hydride offers the potential of up to 7.6 wt.% hydrogen, one of the highest capacities of any metal hydride. However, the high stability of the hydride phase necessitates operation at elevated temperatures and the addition of catalysts, both of which reduce the overall hydrogen storage density. Researchers at ITRI have developed catalysts that can improve kinetics considerably, which will be combined with the thermal analysis done by Purdue to produce a feasible system.

Metal Hydride Heat Pump
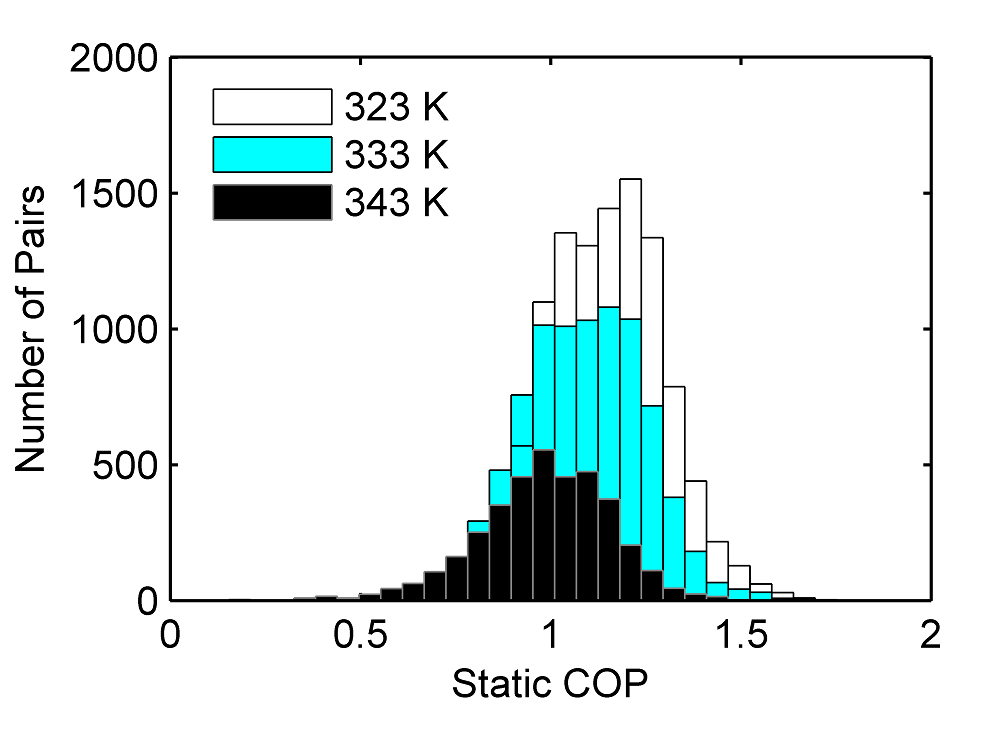
An object-oriented metal hydride MATLAB toolbox has been developed to help create more robust models for thermodynamic equilibrium, reaction rates, and thermophysical properties. During its development, a database of over 300 hydrides was assembled and identified over 1800 hydride pairs with coefficients of performance (COP) greater than 1. Lastly, we used a hydride pair scoring system to help focus on top pairs.