Interaction of Chloride-based Deicing Salts with Concrete (cont. C)
MgCl2 (hexahydrate - MgCl2 . 6H2O)

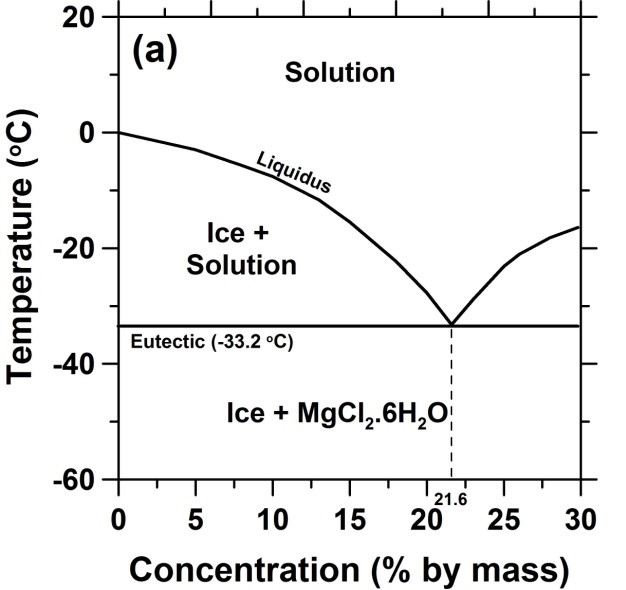
As shown in the statistics section, magnesium chloride can provide the largest freezing point of the four deicers, for solution concentrations less than about 20 %. However, magnesium cations can be very damaging to concrete due to their reaction with hydrated portland cement paste to produce magnesium-silicate-hydrate gel, brucite, and magnesium oxychloride compounds; in extreme cases, such reactions can effectively turn the concrete into mush. As magnesium cations are replacing calcium cations during this reaction, all of the reactions present in the case of CaCl2 deicing salt also come into play when MgCl2 is employed in the field. The phase diagram of MgCl2 in water is shown below.
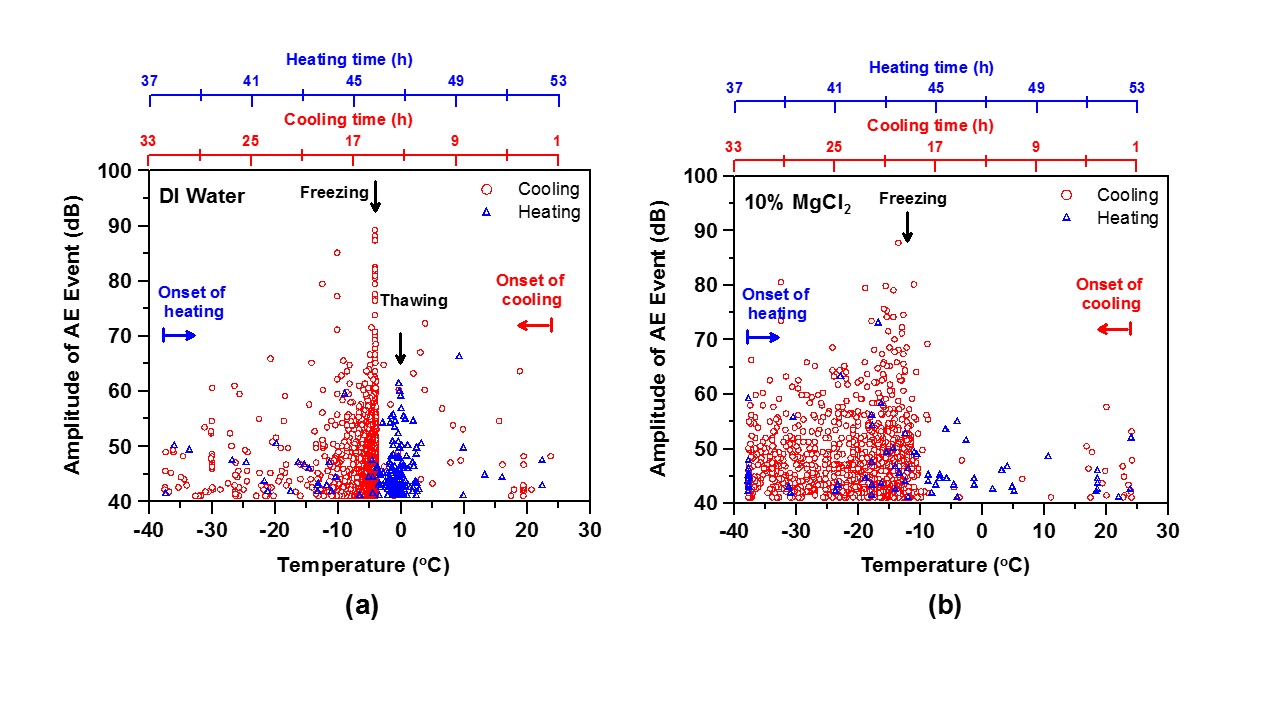
The acoustic emission measurements performed using the AE-LGCC are shown in the figure below for saturation with distilled water and with a 10 % solution of MgCl2. Significant damage is indicated in both cases.
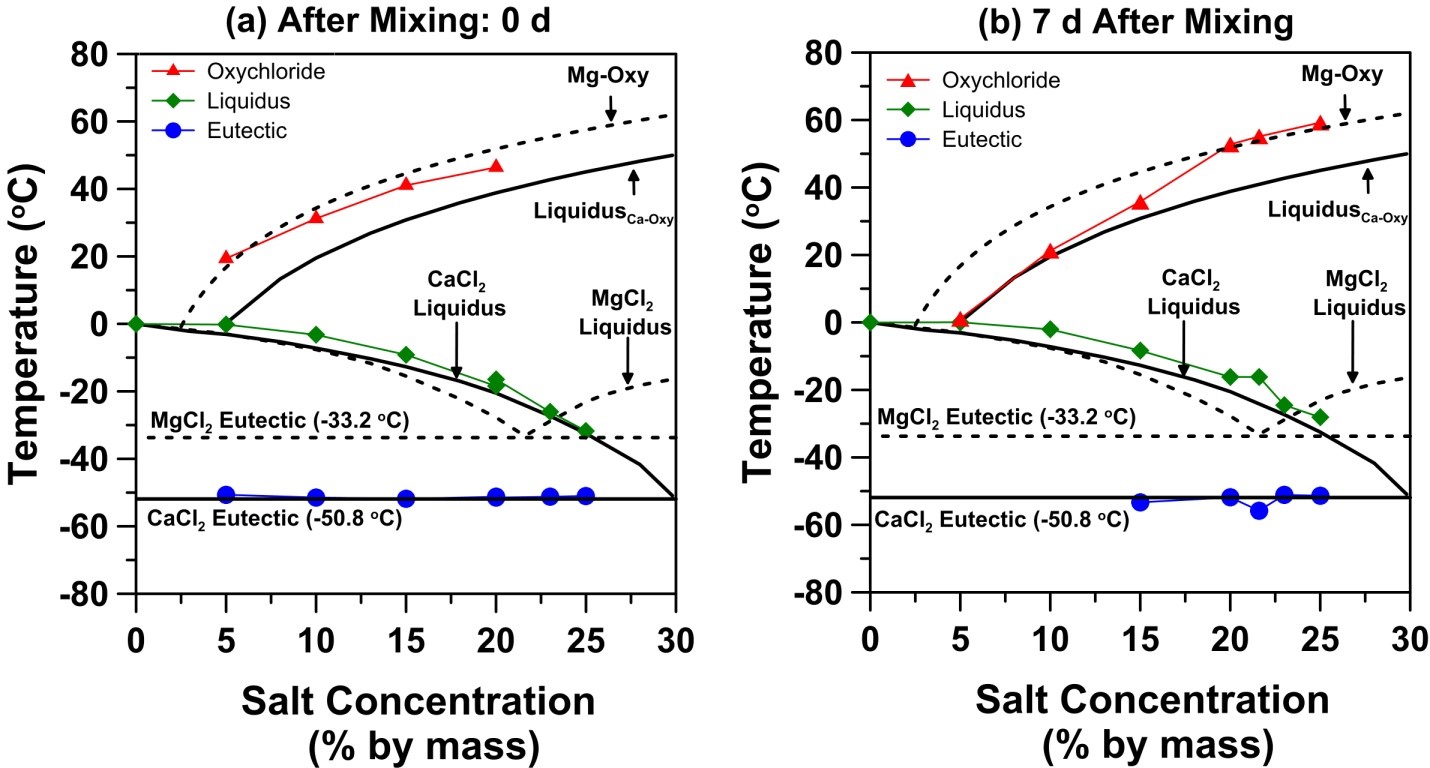
The results lead to an updated phase diagram for the MgCl2-cement-water system as shown below, determined using LT-DSC after the MgCl2 solution is mixed directly with hydrated cement powder.
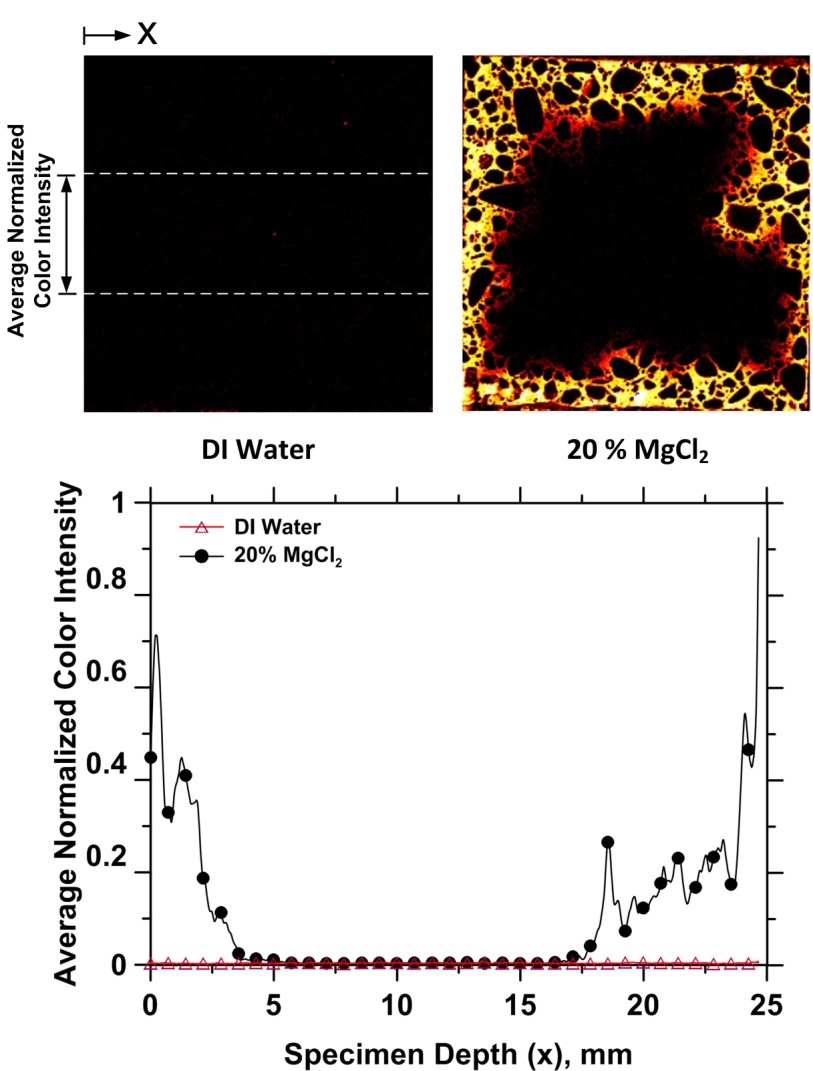
Once again, as with CaCl2, higher concentration MgCl2 solutions produce extensive precipitation and clogging of the mortar specimens pores so that even under vacuum saturation conditions, the Cl- ions are only able to penetrate a short distance into the specimen (see XRF figure and profile below) and the interior of the specimen remains only partially saturated.
Interaction of Chloride-based Deicing Salts with Concrete:
NaCl - the most well-established road salt
CaCl2 - (dihydrate - CaCl2 . 2H2O)