Interaction of Chloride-Based Deicing Salts with Concrete
| Author: | Farnam, Y, Bentz, D.P., and Weiss, W.J., |
|---|
These salts are necessary to provide safe winter driving conditions and save lives by preventing the freezing of a layer of ice on our nation's roads and bridge decks. However, the safety and sense of comfort provided by these salts is not without a price, as these salts can greatly contribute to the degradation and decay of reinforced concrete transportation systems. Most salts are chloride-based and when the applied salts diffuse into the concrete and reach the level of the steel reinforcement, the chloride ions can quickly depassivate the steel and activate corrosion reactions that can ultimately result in the loss of functionality of the concrete structure. Furthermore, research has indicated that these same salts attack the concrete itself, through reactions and phase changes, producing dimensional changes and cracking of the concrete. The further penetration of the salts into these cracks sets up a vicious cycle of concrete spalling and degradation. The goal of the current study has been to develop a toolbox of analysis techniques to better understand these reactions and phases changes and the damage that they produce in concrete at a fundamental level.
Some Statistics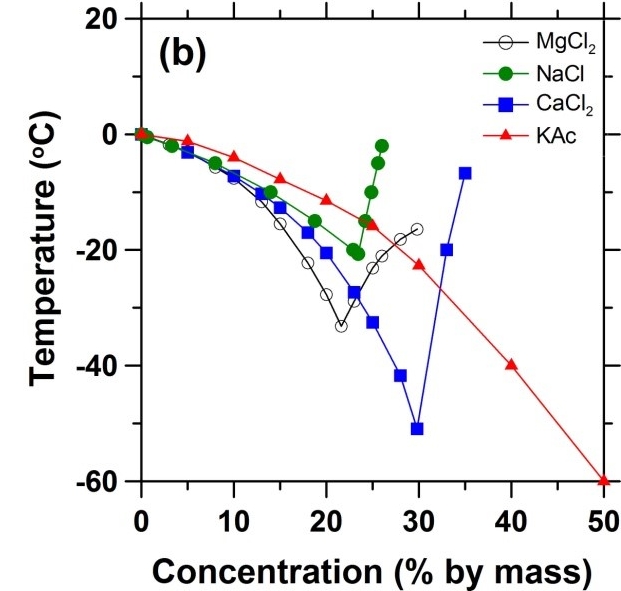
According to the Alexandria, VA based Salt Institute, about 17 million tons of deicing salt is applied annually to U.S. roadways. That works out to about 100 lbs. of road salt per person in the U.S. According to the Salt Institute website, a Marquette University study determined that de-icing roads with salt reduces accidents by 88 % and injuries by 85 % during winter storms. From year to year, although much of this salt is washed off into our nation's waterways (potentially causing problems for wildlife, etc.), there is a considerable accumulation of salt within the concrete roadway or bridge deck.
The right image contrasts the freezing-point depression of different deicing salts, including the three from the present study along with potassium acetate (KAc). While MgCl2 provides the highest freezing point depression for a 20 % concentration solution, for the three chloride-based salts, the largest depression overall (below -50 oC) can be achieved at the eutectic point (30 %) for the CaCl2 salt!
 The Toolbox
The Toolbox
Acoustic emission low temperature longitudinal guarded comparative calorimeter (AE-LGCC)- Provides a controlled cooling/heating rate environment and measures the heat flow within (via temperatures) and damage of (via acoustic emission) cement-based materials (typically mortar prisms) during freezing/thawing cycles.
Isothermal calorimeter - Measures the heat produced by reactions at a constant temperature and in this study is used to assess reactions between deicing salts and cement-based materials and components (such as pore solution or calcium hydroxide).
Low temperature differential scanning calorimeter (LT-DSC) - measures the heat flow absorbed/released in a small specimen during a controlled cooling/heating experiment. Provides a higher degree of sensitivity than the AE-LGCC equipment for mapping out phase diagrams and assessing heat capacity as a function of temperature.
X-ray fluorescence (XRF) imaging - Provides a two-dimensional mapping of the elemental distribution at the surface (as received, broken, or sliced) of a specimen. In this study, image maps and profiles of the Cl- distribution are produced to better understand the interaction of the deicing salt with the cement-based material.
Interaction of Chloride-based Deicing Salts with Concrete:
NaCl - the most well-established road salt
CaCl2 - (dihydrate - CaCl2 . 2H2O)
MgCl2 - (hexahydrate - MgCl2 . 6H2O)
Take Away Messages
- Chloride-based commercial deicing salts strongly interact with the concrete to which they are applied, in addition to their desirable characteristic of freezing point depression.
- The interactions between the salts and phases present in the hydrated cement paste in the concrete can produce additional phases and phase changes that when expansive in nature can cause additional cracking and damage to the concrete.
- In the present study, of the three chloride-based road salts that were examined, NaCl appears more benign than either CaCl2 or MgCl2 with respect to reacting with the concrete substrate.
- The MgCl2 and CaCl2 salts exhibit some similar characteristics, as Ca++ ions are released as the magnesium reacts with the cement phases. For example, both salts produced a densification of the surface due to precipitation that was sufficiently extreme to prevent vacuum saturation from penetrating to the center of the 25.4 mm x 25.4 mm x 50.8 mm mortar prisms employed in the present study.
References
- Farnam, Y., Bentz, D.P., Sakulich, A., Flynn, D., and Weiss, W.J., Measuring Freeze and Thaw Damage in Mortars Containing Salt Using a Low Temperature Longitudinal Guarded Comparative Calorimeter and Acoustic Emission (AE-LGCC), Advances in Civil Engineering Materials,3(1), 23 pp., 2014.
- Farnam, Y., Bentz, D.P., Hampton, A., and Weiss, W.J., Acoustic Emission and Low Temperature Calorimetry Study of Freeze and Thaw Behavior in Cementitious Materials Exposed to Sodium Chloride Salt, Transportation Research Record: Journal of the Transportation Research Board, No. 2441, Concrete Materials 2014, 81-90, 2014.
- Farnam, Y., Dick, S., Wiese, A., Davis, J., Bentz, D.P., and Weiss, W.J., The Influence of Calcium Chloride Deicing Salt on Phase Changes and Damage Development in Cementitious Materials, submitted to Cement and Concrete Composites, 2014.
- Farnam, Y., Wiese, A., Bentz, D., Davis, J., and Weiss, J., Damage Development in Cementitious Materials Exposed to Magnesium Chloride Deicing Salt, Construction and Building Materials, 93, 384-392, 2015.
- Farnam, Y., Washington, T., and Weiss, J. (2015), The Influence of Calcium Chloride Salt Solution on the Transport Properties of Cementitious Materials, Journal of Advances in Civil Engineering, Vol. 2015, pp. 1-13, Hindawi. doi: 10.1155/2015/929864.
