Interaction of Chloride-based Deicing Salts with Concrete (cont. B)
CaCl2 (dihydrate - CaCl2 . 2H2O)
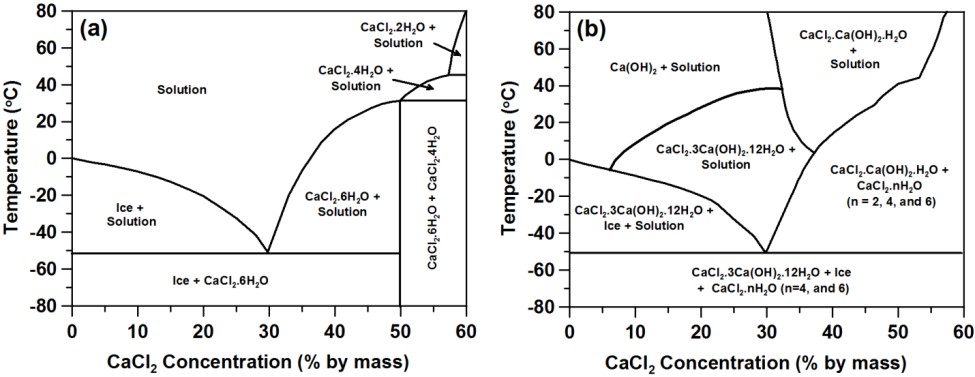
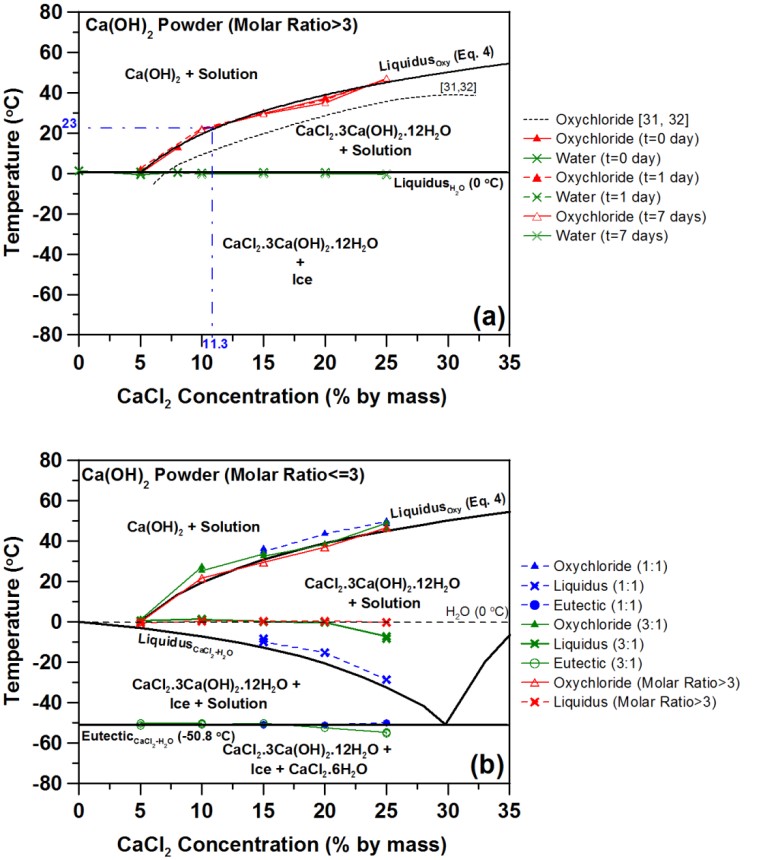
As shown in the statistics section, calcium chloride can provide a larger freezing depression than its sodium counterpart. The phase diagram for CaCl2 is shown below, without (left) and with (right) the presence of calcium hydroxide (which leads to the formation of calcium oxychloride phases). In the image on the right, the phase diagram shown is valid for molar ratios of calcium hydroxide to calcium chloride less than or equal to 0.5. Once again, formation of these calcium oxychloride phases can be expansive and damage the concrete even when no freezing (of water) occurs, as indicated by the acoustic emission measurements shown in the second set of images below. At room temperature, calcium oxychloride will form if the CaCl2 concentration of the solution exceeds about 11.3 %, as the reaction between calcium hydroxide (in the mortar) and the CaCl2 solution is very rapid (as verified by isothermal calorimetry measurements, not shown).
For higher molar ratios of calcium hydroxide to calcium chloride (> 3), the phase diagram is modified significantly as shown below, as there is then sufficient calcium hydroxide present to convert all of the CaCl2 to oxychloride.
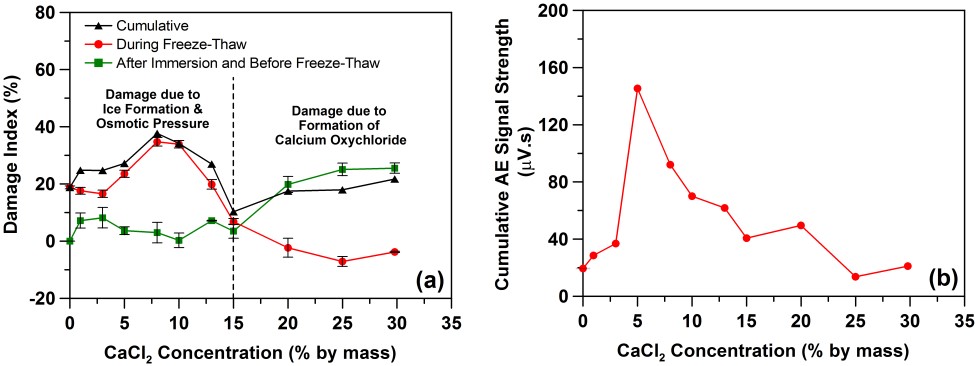
As shown in the figure on the left below, measurements of elastic modulus and computation of a damage index as the relative change in elastic modulus from the original value indicate that substantial damage occurs to the mortar specimens during saturation with the higher concentrations of CaCl2 solutions due to the formation of calcium oxychloride. For the conditions and procedures utilized in this study, a pessimism concentration of about 5 % to 10 % CaCl2 is found in terms of maximizing the acoustic emission signal strength and the modulus-based damage index (right image below).
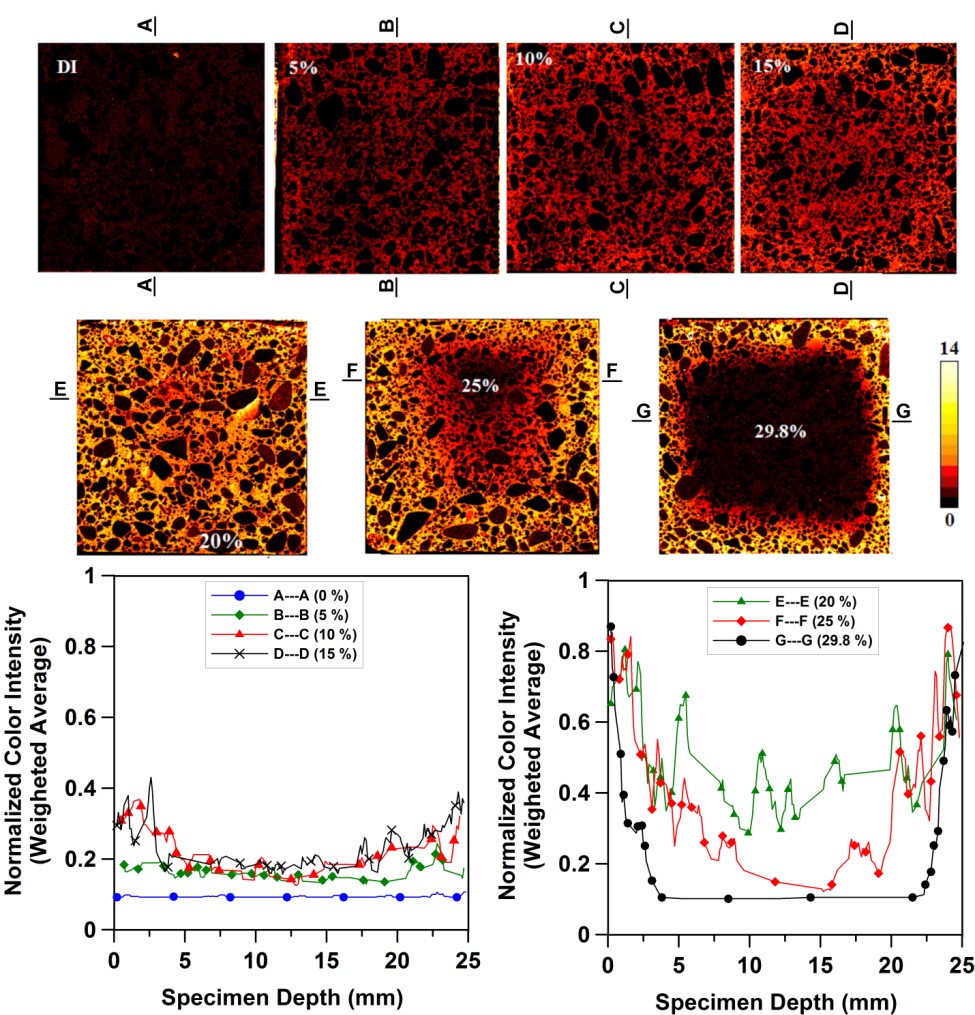
In this case, XRF imaging indicates an additional consequence of the oxychloride formation, mainly that it densifies the surface microstructure of the mortar specimens to the point where even under vacuum saturation conditions, the penetration of Cl- ions to the center of the specimen is severely limited (see the 25 % and 29.8% images and their accompanying profiles below).
Interaction of Chloride-based Deicing Salts with Concrete:
NaCl - the most well-established road salt
MgCl2 - (hexahydrate - MgCl2 . 6H2O)