Research Areas
Overview
Research in the Kinzer-Ursem lab focuses on developing tools, both computational and experimental, to advance quantitative descriptions of biomolecular processes and disease within two areas of expertise: 1) biomolecular technologies for detection of disease biomarkers, and 2) computational modeling of biological and biomedical systems. Results from each of these areas of investigation are integrated and inform new studies in the other area. For example, the need for quantitative data to parameterize computational models has led to the development and application of particle diffusometry technology. Predictions from computational models guide the development of assays for measuring protein interactions and imaging protein complexes in situ. Results from protein assays and imaging experiments guide model development and lead to new questions about mechanisms that underlie the biological and biomedical systems under study. Through this unique computational-experimental framework our work spans the entire spectrum of fundamental theory and simulation to experimental implementation to translational work in field studies with collaborators.Spatial Stochastic Modeling of Protein Network Dynamics
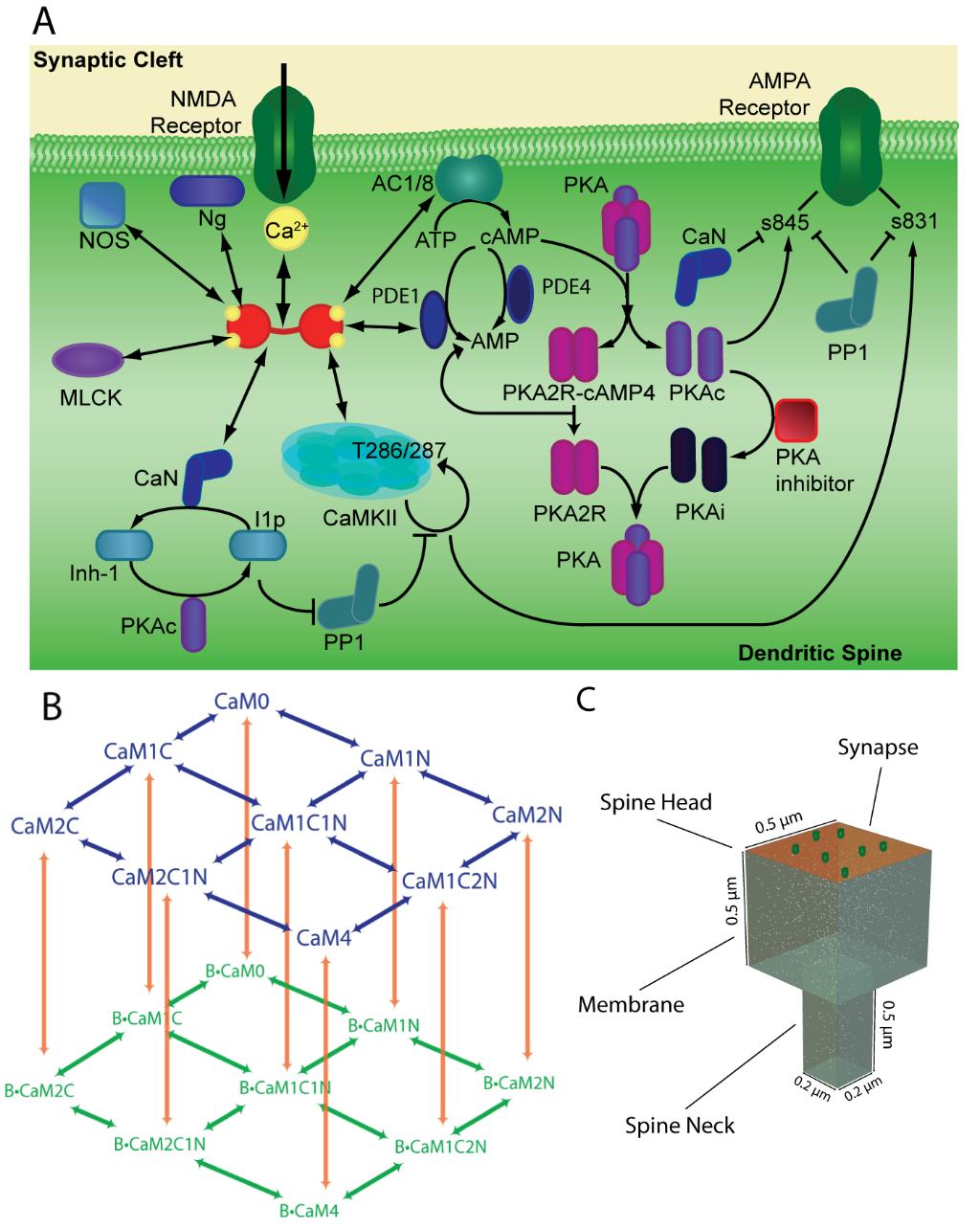
Calcium-dependent protein signaling regulates a variety of cellular process ranging from insulin secretion, cell motility, and cardiac muscle contraction. Particularly in learning and memory, calcium-dependent protein pathways are involved in regulating the connective strength between neuronal synapses. We use computational modeling to quantitatively describe calcium second messenger signaling in dendritic spines; cellular compartments on excitatory neurons that are important for learning and memory. We have described a new protein signaling mechanism, called “competitive tuning” that may be an important mechanism in protein signaling network function; akin to spatial localization and feedback control with significant implications in cell behavior and drug target analysis. We are developing a spatial stochastic computational model to study how calcium signaling pathways are sensitive to these temporal waves of calcium that diffuse with spatial gradients in the dendritic spine. These short time-scale spatial gradients affect the temporal tuning of protein activation by Ca2+/CaM, and in turn, regulate the induction of synaptic plasticity.
Mechanistic Computational Modeling of Implantable, Bioresorbable Drug Release Systems
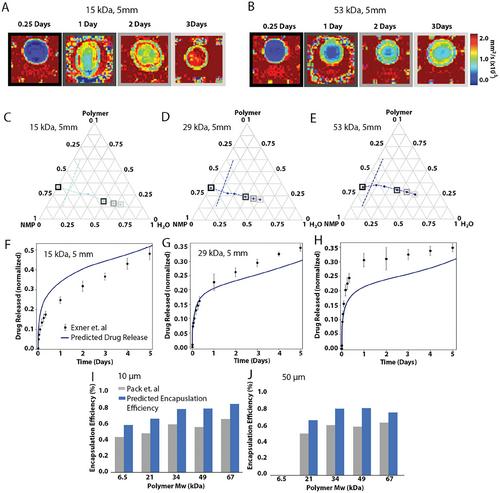
Implantable, bioresorbable drug delivery systems offer an alternative to current drug administration techniques; allowing for patient-tailored drug dosage, while also increasing patient compliance. Mechanistic mathematical modeling allows for the acceleration of the design of the release systems, and for prediction of physical anomalies that are not intuitive and may otherwise elude discovery. This study investigates short-term drug release as a function of water-mediated polymer phase inversion into a solid depot within hours to days, as well as long-term hydrolysis-mediated degradation and erosion of the implant over the next few weeks. Finite difference methods are used to model spatial and temporal changes in polymer phase inversion, solidification, and hydrolysis. Modeling reveals the impact of non-uniform drug distribution, production and transport of H+ ions, and localized polymer degradation on the diffusion of water, drug, and hydrolyzed polymer byproducts. Compared to experimental data, the computational model accurately predicts the drug release during the solidification of implants over days and drug release profiles over weeks from microspheres and implants. This work offers new insight into the impact of various parameters on drug release profiles, and is a new tool to accelerate the design process for release systems to meet a patient specific clinical need.
Portable Disease Diagnostics
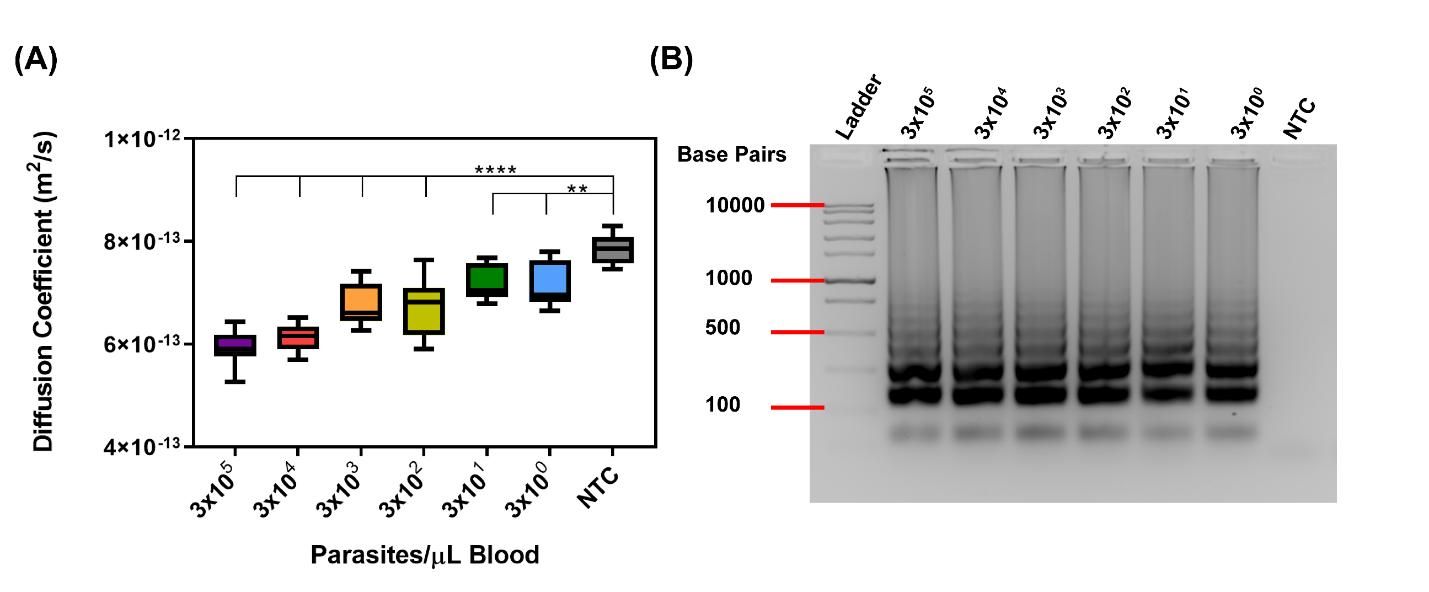
Particle diffusometry (PD) was developed to detect changes in quiescent fluids and is being applied to detect both infectious and chronic disease biomarkers. Developed in collaboration with Dr. Steve Wereley (Purdue, ME), particle diffusometry, has been demonstrated as a sensitive readout to: measure protein conjugation to nanoparticles; detect denaturing of biotherapeutic proteins via changes in solution viscosity; and most impactfully, detect the presence of pathogenic DNA in samples at high sensitivity. Examples include detection of cholera in pond water, malaria in blood samples, and COVID-19 in saliva. More recently we have extended the application of particle diffusometry technology to detect protein-protein interaction at low volume in micro-fluidic devices and to quantitatively measure HIV viral load. With close collaborator Dr. Jacqueline Linnes we recently completed a field study in Eldoret, Kenya were we tested the ability of our hand-held devices to measure the HIV viral load of over 60 patient samples.



Labeling of new protein synthesis in in vivo mouse models
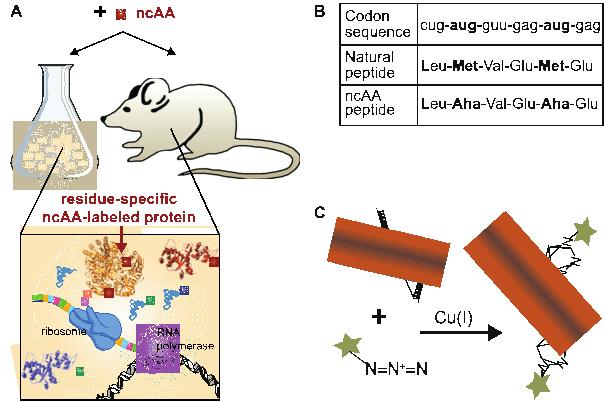
In collaboration with Dr. Sarah Calve (U Colorado, Boulder) we were the first to develop methods to directly administer non-canonical amino acids in vivo in a mammalian model organism for labeling and identification of newly synthesized proteins. These methods have been widely adopted in the matrix biology field to characterize the murine proteome during growth, disease and repair. We have also leveraged our modeling and proteomic skills to describe the biodistribution, uptake, and incorporation of the non-canonical amino acid into proteins in vivo. This work will enable researchers to optimize their injection protocols for maximal tissue labeling.