Research Summary
ETSL focuses on developing a holistic approach toward fundamental understanding of the materials-transport-interface interactions in energy storage and conversion. We specialize in exploring mesoscale physics and stochastics pertaining to physicochemical and reactive transport phenomena coupling electrochemistry and electro-kinetics/statics, functional materials and manufacturing in clean energy. Our current emphasis is on fundamental understanding of mesoscale and interface interactions in electrochemical energy storage and pertinent implications in performance, life and safety.
- Electrochemical energy storage and conversion (Batteries and Fuel Cells)
- Processing/transport/property interactions
- Mesoscale physics and stochastics in functional materials design and manufacturing
Sponsors
ETSL is grateful for the support of its sponsors and partners.

Research Projects
-
Active particles inside the lithium-ion battery electrode experience diffusion induced stress and volume change during intercalation. High-rate or low-temperature operation can cause large concentration gradients resulting in higher probability of microcrack formation and propagation in the active particles and ultimately performance decay. Acoustic emission is a non-destructive technique for the detection of mechanical degradation.
In this work, a computational methodology has been developed, based on a dynamic lattice spring model (DLSM), to study acoustic emission characteristics resulting from intercalation induced microcrack formation in electrodes. This method allows relating the acoustic response with the mechanical damage experienced during lithiation/delithiation in the active particles. Energy released due to brittle fracture is rendered as the major source of acoustic emission response. Predictions from this analysis suggest that during cycling maximum amount of acoustic activity is observed in the first couple of cycles. A phase map has been developed to demonstrate the influence of elastic modulus and damping coefficient on the damage evolution and identify a potential window of reduced mechanical degradation.
-
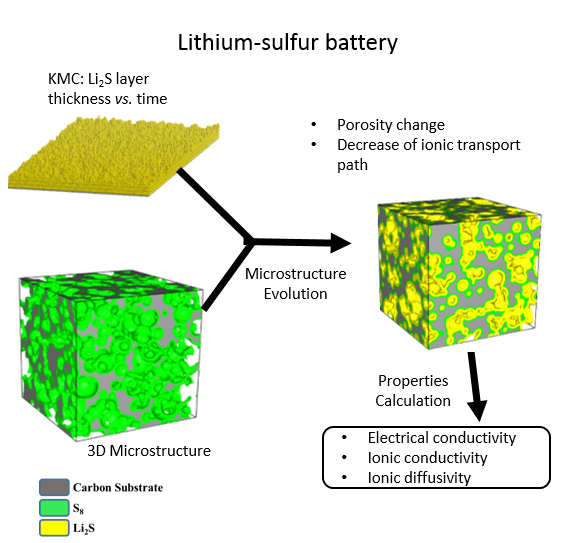
The cathode architecture plays an important role in determining the performance of the Li-S battery. The deposition of Li2S on the substrate affects the porosity of the cathode microstructure, which increases the tortuosity and decreases effective ionic conductivity and diffusivity. In this study, we propose a mesoscale model to fundamentally understand the morphology evolution of Li2S precipitations and the related electrode microstructure variation during the discharge process. In this study, the effect of ambient environment (lithium ion concentration CLi), operating condition (temperature T), and deposit-electrode interaction (reaction barrier G) on the morphology of precipitated Li2S is investigated. A Kinetic Monte Carlo (KMC) model is proposed to investigate Li2S morphology evolution in a large temporal-spatial scale. With the thickness obtained from KMC simulation, the time evolution of the porosity of electrode microstructure can be predicted and reconstructed. The variation of porosity can be affected by the original substrate surface area, pore size, and morphology of discharge product. Moreover, the thickness of discharge product and variation in porosity are a function of lithium ion concentration and temperature. The time evolution of microstructure porosity can further be used to evaluate the effective electrochemical properties, such as effective electrical conductivity, ionic conductivity, and ionic diffusivity.
-
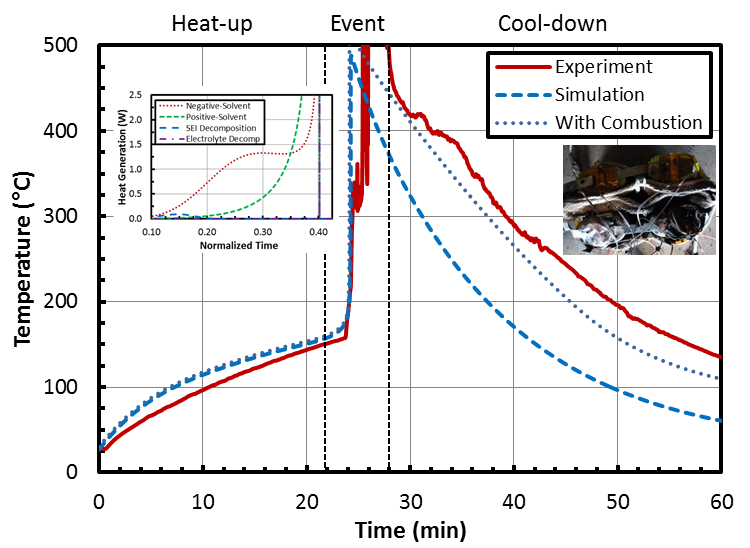
The aim of this study is to determine the effect of various combined active (liquid heat exchanger) and passive (phase change material) thermal management techniques on cell temperatures and thermal/electrical balancing. The cell configuration and volume/weight constraints have important roles in optimizing the thermal management technique, particularly when using both active and passive systems. A computational modeling study including conjugate heat transfer and fluid dynamics coupled with thermo-electrochemical dynamics is performed to investigate design trade-offs in lithium-ion battery thermal management strategies. These strategies were experimentally tested under abuse conditions to validate the models against realistic behavior.
Should the thermal management system fail to regulate cell temperatures, a series of exothermic side reactions can occur that trigger an event called thermal runaway. Due to significant concern with not just the onset of thermal runaway, but with thermal runaway propagation, various concepts were tested to mitigate the propagation of a thermally triggered condition from a source cell to adjacent cells. The electrolyte used in LIBs vaporizes at 150 °C and can subsequently cause cell rupture or fire. This extreme temperature can be reached under abuse conditions such as external/internal short, overcharge, or operation outside of the recommended thermal window. If thermal runaway occurs, it is imperative for the module design to be able to reject enough heat to prevent adjacent cells from also heating excessively and causing a chain reaction.
-
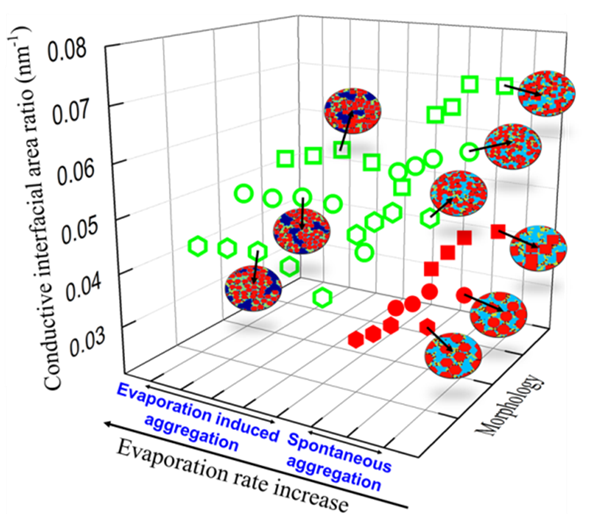
The electrode microstructure and composition play an important role in determining the performance of the LIB. To this end, the processing of the multi-phase slurry consisting of active nanoparticles, conductive additives, binder and solvent determines the electrochemical properties and performance of the electrode.
Fundamentally, slurry properties and drying methods mainly determine the microstructure and the performance of the cathode composite. In our present work, a morphology-detailed mesoscale model has been developed to gain fundamental understating of the influence of active particle morphology, size, volume fraction, solvent evaporation rate, and multi-phase (active particle, conductive additive, binder and solvent) interaction.
-
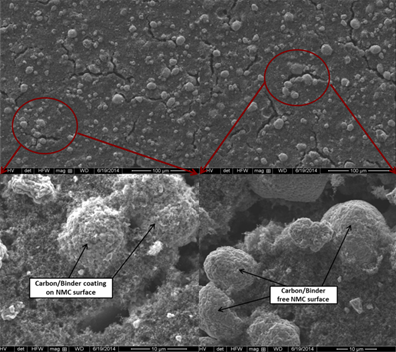
The performance of batteries, regardless of type, is dependent on the materials that form the positive and negative electrode, the choice of electrolyte, and the cell architecture. The effects of varying the composition and chemistry of LIBs are well documented, however a critical aspect that is often overlooked is the processing steps during fabrication.
Typical processing parameters such as calendaring or solvent evaporation have a significant impact on critical parameters such as porosity or constituent distribution within the electrodes, and thus can affect the electrochemical performance. With new materials having the side effect of being extremely expensive, changes to processing steps represent a cost-effective measure for improving the performance of LIBs.
-
The analysis of impedance response of Lithium-ion battery (LIB) electrodes can be useful for predicting cell performance and degradation. In this study, virtual 3-D microstructures of LIB electrodes with intercalation particles are designed to describe the influence of microstructure on the electrochemical impedance response. The technique of digital stochastic modeling has been employed for the generation of electrode microstructures consisting of active material, binder, conductive additive and electrolyte. Physicochemical properties for each of the constituent phases have been duly accounted for. Mathematical models have been developed to characterize the electrochemical impedance of LIB electrode.
The morphology of the active material (i.e. particle size and shape) has been proven to have influence on the side reaction rate and the SEI formation on the active material. Thereby, a systematic investigation of the influence of microstructure design, which varies the particle-size distribution or the shape of active material, on the SEI formation and the corresponding resistance increase is provided in this work. In this regard, a modeling approach combining SEI formation and porous electrode EIS is presented, which predicts the impedance response in the presence of a SEI layer.
In this work, we also demonstrate the coupling of electrode microstructures to the solid state diffusion impedance response in LIB electrodes. This model considers not only the effect of heterogeneity in active particle size on the local bounded diffusion impedance response, but also the effect of interfacial surface area and volume fraction of the active materials in the electrode on the impedance response. It also captures the effect of electrical conductivity on overall impedance response. Additionally, the impact of the morphology of the active materials on the diffusion impedance response through utilization of the characteristic diffusion length of active particles and an effective mean particle size has been demonstrated. This approach is envisioned to offer a virtual impedance response probing framework to elucidate the influence of electrode microstructural variability and underlying electrochemical and transport interactions.