February 11, 2021
Green flash: High-speed infrared helps reveal safer hypergolic propellant
WEST LAFAYETTE, Ind. — When SpaceX’s Crew Dragon capsule splashed down off the Florida coast in August following its first crewed mission, the two astronauts inside could not exit the capsule immediately. Technicians outside had to confirm there were no airborne vapors from hydrazine, a highly toxic fuel used by the vehicle’s hypergolic thrusters.
Now, Purdue University combustion researchers are investigating a safer and less toxic hypergolic propellant, studying its explosive reaction with a new technique involving both visible and infrared high-speed cameras. Hypergolics are substances that instantly ignite when they come into contact with each other.
“Hypergolics have been used all the way back to the Apollo era and before,” said Steven Son, Purdue’s Alfred J. McAllister Professor of Mechanical Engineering, and an expert in energetic materials such as propellants, explosives and pyrotechnics. “They can be stored at room temperature, and they instantly ignite when mixed, which makes them more versatile and reliable than cryogenic fuels.”
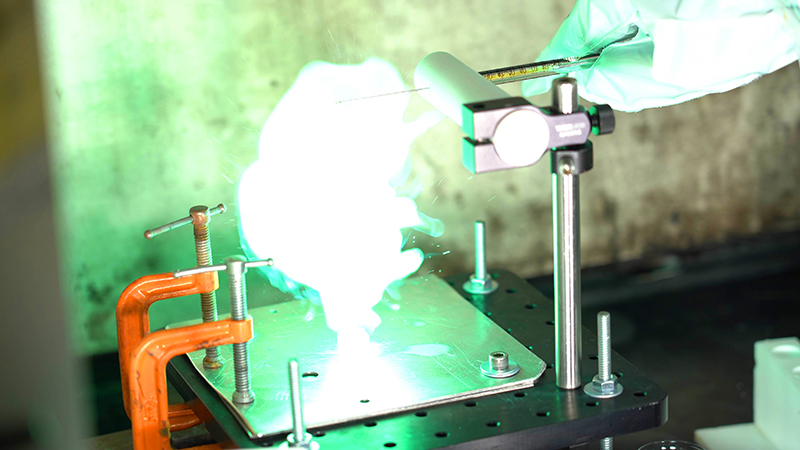 Ammonia borane is a hypergolic propellant that releases a brilliant green flash when exposed to an oxidizer. Purdue researchers are developing ammonia borane as a safer alternative to current hypergolic fuels. (Purdue University photo/Jared Pike)
Ammonia borane is a hypergolic propellant that releases a brilliant green flash when exposed to an oxidizer. Purdue researchers are developing ammonia borane as a safer alternative to current hypergolic fuels. (Purdue University photo/Jared Pike)
Although their use in rocketry is well-documented, current hypergolic fuels also are notoriously dangerous for humans to handle, and bad for the environment.
“We want to develop hypergolic fuels that have good performance, but are also much less toxic,” Son said. “And that’s why we have been experimenting with ammonia borane.”
Unlike most other hypergolic fuels, ammonia borane (NH3BH3) is a solid material, stable in typical atmospheric conditions. Because of its hydrogen density, it was first developed as a solid-state storage medium for hydrogen. But combustion researchers have recently discovered its hypergolic properties, which could be used as part of a hybrid propellant.
“Before this can be used in the real world, we have to understand the fundamental combustion science governing its behavior,” said Chris Goldenstein, a Purdue assistant professor of mechanical engineering. “We are using a new approach that combines visible and infrared imaging to characterize the combustion process.”
Their work has been published in Proceedings of the Combustion Institute.
Infrared imaging allows researchers to see the chemical composition of the flame throughout the combustion process.
“Every molecule has a unique spectral fingerprint,” Goldenstein said. “By looking for specific wavelengths of light, we can identify where in space certain molecules are distributed and know how complete the combustion process is. Many of the desired wavelengths are not visible to the naked eye, and infrared imaging is the only way to see them.”
Because the reaction takes place in just a few milliseconds, the researchers use specialty cameras capable of capturing at least 2,000 frames per second. The high-speed video reveals a remarkable and rapidly expanding green flash, demonstrating the power of hypergolic substances.
“We generally start with very small samples,” said Michael Baier, a Ph.D. student in Purdue’s School of Aeronautics and Astronautics, who conducts the experiments at Zucrow Labs. “We use just a little powder of the ammonia borane, and above it is a syringe that dispenses a microliter droplet of the oxidizer, which, in this case, is white fuming nitric acid. Even then, it makes a pretty big bang. Those few milliseconds give us all the data we need to characterize the ignition.”
Son said, “Thanks to infrared imaging, we saw a lot of BO2 signal, which was surprising to us. This indicates that ammonia borane is achieving complete combustion even better than conventional boron fuels.”
Although ammonia borane may be less toxic than traditional hydrazine-based hypergolics, it’s still fairly dangerous to work with, as are all energetic materials. But Zucrow Labs has been researching propulsion technologies since 1948 and is one of the few labs in academia that is fully equipped to study energetic materials.
“To be able to handle these materials safely and work with these kinds of propellants is unique,” Son said. “Purdue is one of the few places that is able to do this kind of research.”
Zucrow’s capabilities are part of the reason why the Army Research Lab chose Purdue for a multimillion-dollar collaborative research agreement to modernize the U.S. Army’s modeling, manufacturing, usage and disposal of energetic materials.
“Combustion is a complex process involving hundreds of chemical compounds,” Goldenstein said. “Each one tells their own unique story about the reaction sequence. With this method, we can see those individual stories.”
This work was supported through funding provided by the Army Research Office through grant W911NF-15-1-0201, the National Science Foundation Graduate Research Fellowship program (grant DGE-1333468), and a NASA Space Technology Research Fellowship (grant 80NSSC17K0177).
About Purdue University
Purdue University is a top public research institution developing practical solutions to today’s toughest challenges. Ranked the No. 5 Most Innovative University in the United States by U.S. News & World Report, Purdue delivers world-changing research and out-of-this-world discovery. Committed to hands-on and online, real-world learning, Purdue offers a transformative education to all. Committed to affordability and accessibility, Purdue has frozen tuition and most fees at 2012-13 levels, enabling more students than ever to graduate debt-free. See how Purdue never stops in the persistent pursuit of the next giant leap at https://purdue.edu/.
Media contact: Kayla Wiles, 765-494-2432, wiles5@purdue.edu
Writer: Jared Pike
Sources: Chris Goldenstein, csgoldenstein@purdue.edu
Steve Son, sson@purdue.edu
Journalists visiting campus: Journalists should follow Protect Purdue protocols and the following guidelines:
- Campus is open, but the number of people in spaces may be limited. We will be as accommodating as possible, but you may be asked to step out or report from another location.
- To enable access, particularly to campus buildings, we recommend you contact the Purdue News Service media contact listed on the release to let them know the nature of the visit and where you will be visiting. A News Service representative can facilitate safe access and may escort you on campus.
- Correctly wear face masks inside any campus building, and correctly wear face masks outdoors when social distancing of at least six feet is not possible.
ABSTRACT
High-speed multi-spectral imaging of the hypergolic ignition of ammonia borane
Michael J. Baier, Austin J. McDonald, Kathryn A. Clements, Christopher S. Goldenstein, Steven F. Son
DOI: 10.1016/j.proci.2020.08.025
Recently, ammonia borane (a hydrogen-dense solid fuel) has received increased attention as a potential fuel for use in hybrid rocket systems due to its potential for higher performance. Ammonia borane (AB) is also highly hypergolic with white fuming nitric acid (WFNA) while lacking the health hazards associated with more conventional hypergolic fuels (e.g. hydrazine and its derivatives). To date, there remains a lack of information regarding the combustion behavior of ammonia borane. This work makes use of simultaneous visible and infrared multi-spectral imaging to study the combustion behavior of AB during a hypergolic ignition event (with reagent grade nitric acid droplets applied to the AB as the oxidizer). Using optical filters corresponding to previously reported spectral bands, emission from BO, BO2, HBO2, and the B-H stretch mode of AB were selectively imaged. A two-step ignition process consisting of an initial gas evolution step followed by a separate, seemingly premixed flame propagation step was observed. Observation of HBO2 emission during the pre-ignition/gas evolution phase suggests that rapid formation of HBO2 occurs even at the lower temperatures present prior to ignition. This initial formation of HBO2 (and the subsequent heat release associated with it) may be a major driver of AB/nitric acid decomposition. Emission intensity profiles obtained after the AB samples began burning with the ambient air suggests some degree of HBO2 consumption within the flame front, seemingly counter to the current understanding that HBO2 is a stable product species. This may indicate that boron combustion via boranes may proceed to more complete combustion than is typical for more conventional boron fuels. The ignition dynamics and sequence of boron product formation was well-captured by the multi-spectral imaging technique applied to this reaction system for the first time. This technique could be applied for further study of other boron systems.
Note to journalists: A video about the hypergolics research is available on YouTube. For a copy of the paper, please contact Kayla Wiles, Purdue News Service, at wiles5@purdue.edu or 765-494-2432. Photos, video and other multimedia of the “green flash” experiments are available via Google Drive. Journalists visiting campus should follow visitor health guidelines.

