ME 200 – Thermodynamics I – Spring 2020¶
Homework 2: Systems, Definitions¶
Part (i): A biker¶
Part (ii): A piston-cylinder with heater¶
Part (iii): Thermodynamic definitions¶
Part (i): A biker¶
Given:¶
A biker and bike traveling down the road.
Find:¶
Draw a system with a boundary, indicate whether the system is closed or open, and depict all of the energy and mass flows crossing the boundary of the system for two cases: a) The biker is the system, b) The bike is the system.
System Sketch:¶

Solution:¶
(a) The biker is an open system. Mass flows crossing the boundary of the system include: 1) air and moisture entering due to breathing, 2) carbon dioxide, nitrogen, moisture, etc. leaving due to breathing, 3) moisture leaving due to "sweat". There is a net work output from the biker: the biker is doing work on the bike through petaling and against drag forces, while the bike is returning work to the biker from the petals. There is heat transfer from the biker to the surroundings due to a temperature difference that depends on the bikers metabolic rate. Dots appear above the symbols in the diagram below to indicate "rates" of transfer (e.g, kW) that are continually occurring.

(b) The bike material is a closed system. It is difficult to draw the boundary, but the system would include the frame, gears, pedals, tire/wheels, etc. There is a net work input from the biker: the biker is doing work on the bike through pedaling, while the bike is returning work to the biker from the petals and also working against drag and frictional forces on the bike. There would also be a relatively small amount of heat transfer from the bike to the surroundings due to temperature differences resulting from frictional dissipation. There could also be some small heat transfer between the biker and the bike. Dots appear above the symbols in the diagram below to indicate "rates" of transfer (e.g., kW) that are continually occurring.

Part(ii): A piston-cylinder with heater¶
Given:¶
A piston-cylinder device containing air and an electric resistance heater. The heater uses electrical work (W) as an input and produces a heat transfer (Q) as an output. Through the process of heat transfer to the air, the air expands and pushes the piston upwards against atmospheric pressure above. Neglect any heat transfer from the air to cylinder and piston.
Find:¶
Draw a system with a boundary, indicate whether the system is closed or open, and depict all of the energy and mass flows crossing the boundary of the system for the following cases:
a) The air in the cylinder is the system.
b) The electrical resistance heater is the system.
c) The combination of the air and resistance heater is the system.
d) The piston is the system.
System Sketch:¶

Solution:¶
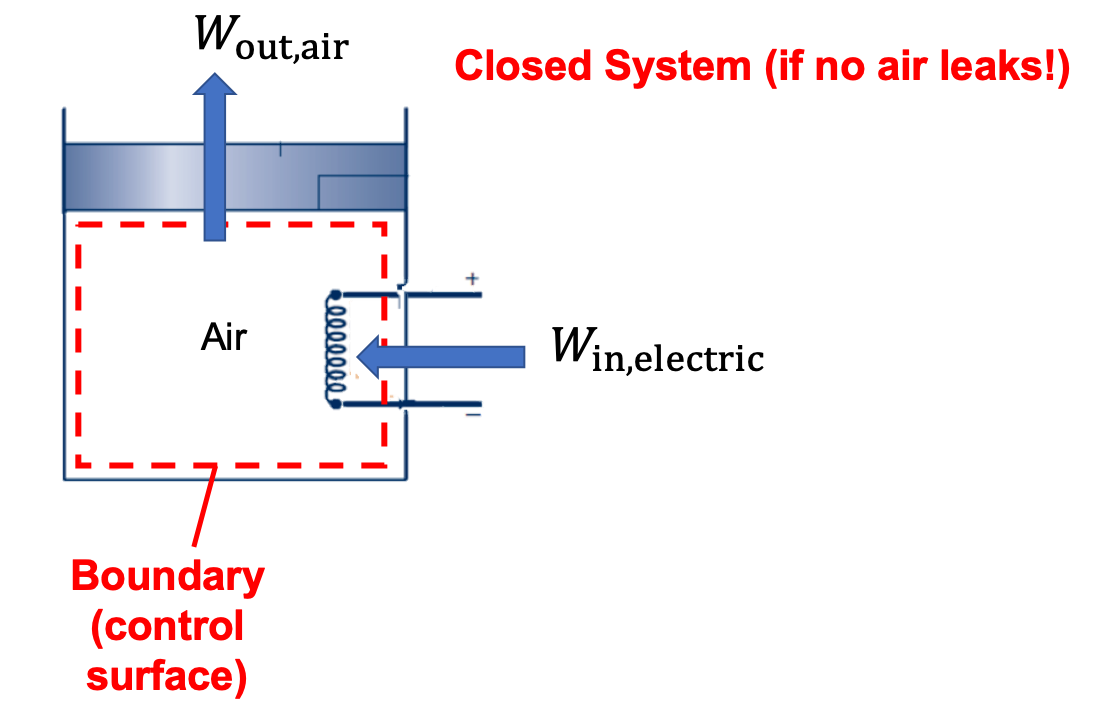
(a) The air in the cylinder is a closed system since no mass crosses the boundary. There is a heat transfer from the electric resistance heater to the air. As a result of the air expanding, there is mechanical (boundary) work done by the air on the piston. In this case, there are no dots above the symbols in the diagram below because Q and W are energy quantities (kJ) over a finite period where the process occurs.
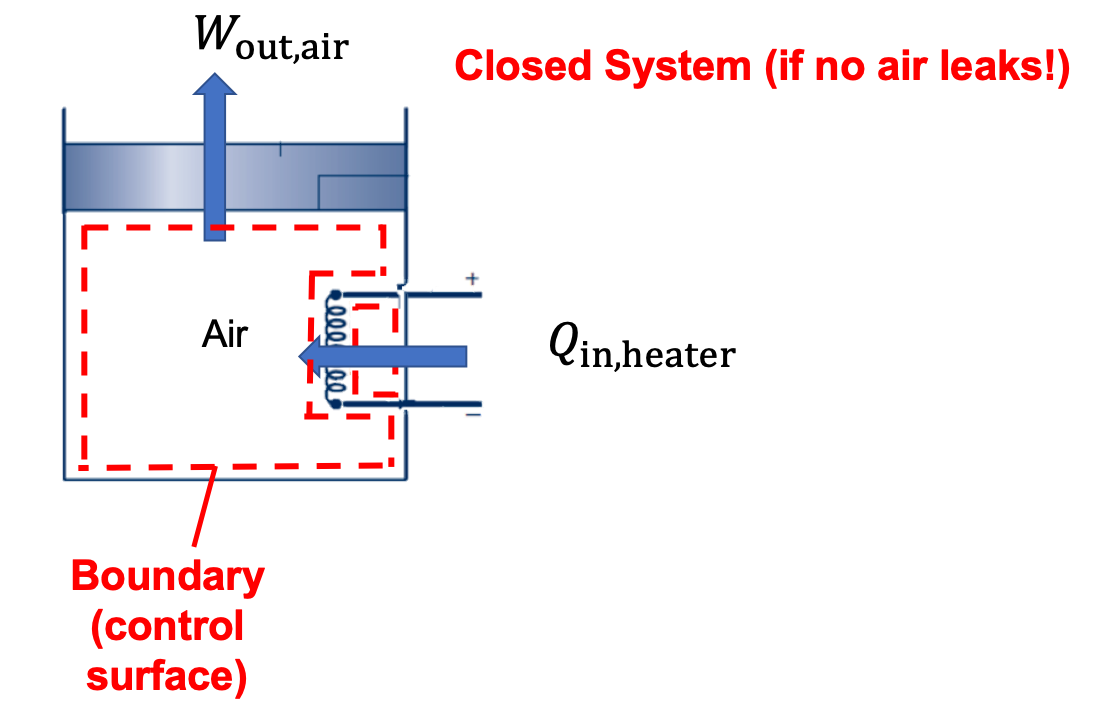
(b) The electrical resistance heater is a closed system. There is electrical work input to the resistor (current flow across a voltage) and there is heat tranfer out of the resistor to the air.
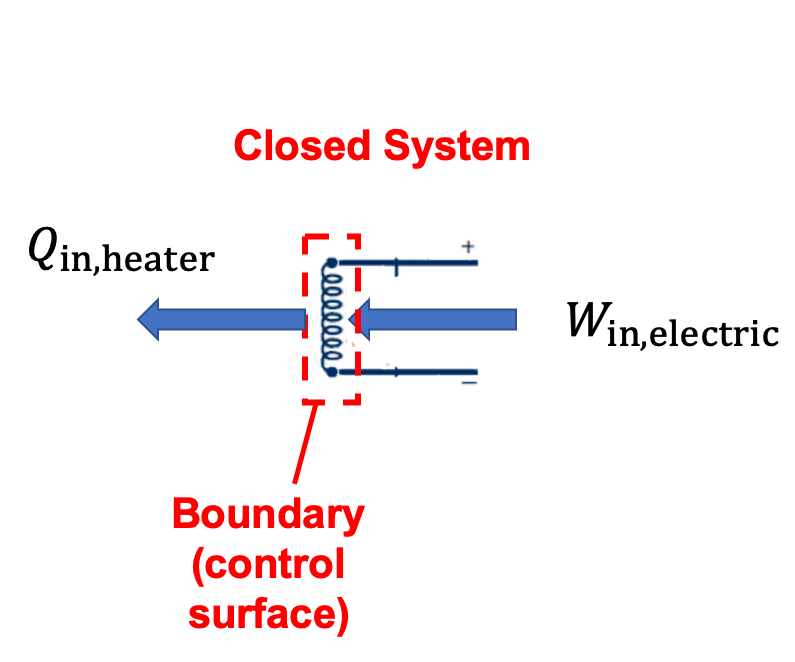
(c) The combination of the air and resistance heater is also a closed system. In this case, electrical work and piston work cross the boundary of the system. The heat transfer betweeen the resistor and air is within the boundaries of the system and should not be shown.
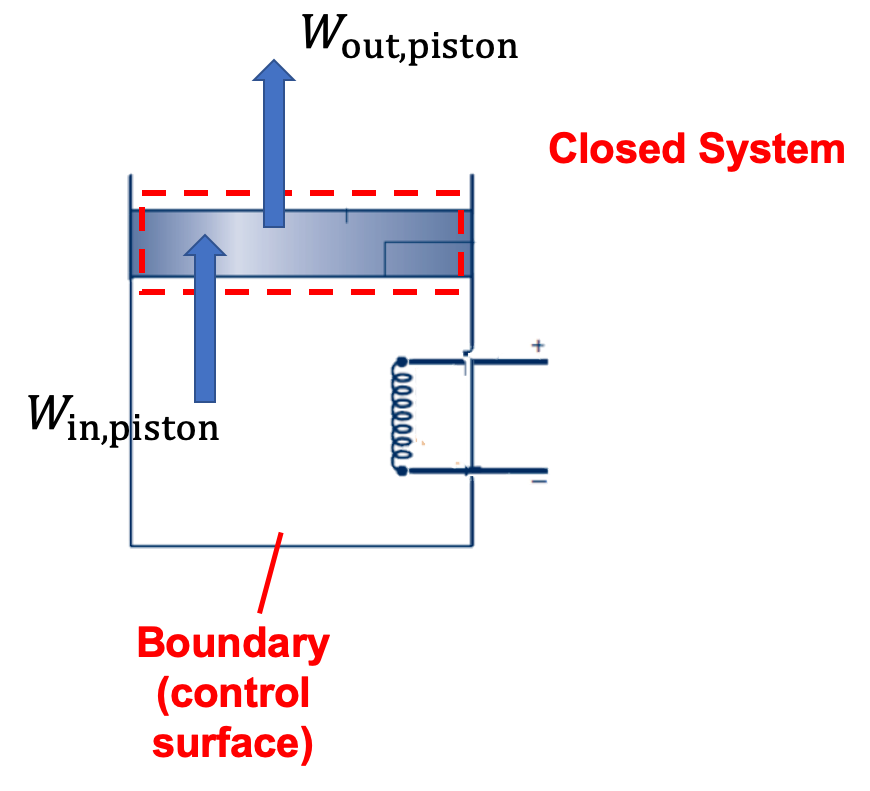
(d) The piston is a closed system. There is a work input done on the piston by the expanding air and the piston does work against the external atmospheric pressure. The difference between the work input and output is the change in potential energy of the piston, which is often negligible compared to the work.
Part (iii): Thermodynamic definitions¶
Solution:¶
(a) Control Volume: A region of space through which mass may flow
(b) Property: A macroscopic characteristic of a system to which a numerical value can be assigned at a given time without knowledge of the previous behavior of the system
(c) Intenive property: A property whose value is independent of the size or extent of a system
(d) State: The condition of a system as described by its properties
(e) Equilibrium state: Condition where when a system is isolated from its surroundings there are no observable changes in properties over time
(f) Process: Change of system from one equilibrium state to another
(g) Quasi-equilibrium process: Slow process where the system is always infinitesimally close to equilibrium at each state along the path;
(h) Path: Series of states for a process