Transition to Turbulence in Stenotic Blood Vessels
Background
Atherosclerosis, a cardiovascular disease of the larger arteries, is the primary cause of
heart disease and stroke. In the United States alone, statistics released by the American
Heart Association estimated that more than 70 million Americans have one or more forms of
cardiovascular disease, with coronary heart disease being the single leading cause of death,
claiming almost 20% of all deaths in 2002 alone. Atherosclerosis is a progressive
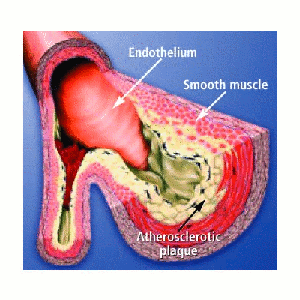
disease initiated by localized fatty streak lesions within the arteries occurring as early as
childhood. Over decades, these lesions can develop into more complex plaques large enough to
significantly block blood flow within the circulatory system. This local
restriction of the artery is known as an arterial stenosis. Plaque deposition is most common
in the aorta, coronary arteries, and carotid arteries; and, as one might expect, the presence
of a stenosis can lead to serious health risks.
Stenoses are commonly characterized as a percentage reduction in diameter or area of the host vessel and are considered clinically significant when the reduction is greater than 75% by area. The progression of a low-level arterial blockage into a critical stenosis is in itself the result of complex non-linear interactions between factors such as flow conditions, wall compliance, and biological responses. The image below shows a cartoon of an arterial stenosis.